Addressing a natural process such as environmental corrosion or material degradation, in which materials in contact with the environment undergo a progressive deterioration over time, means facing a public safety issue. This natural situation worsens when atmospheric and environmental conditions deteriorate; it mainly affects metal-based materials. The consequences of such degradation are dangerous, as they represent not only an economic loss, but also have social and national security implications, while at the same time fostering greater environmental damage.
Environmental corrosion is a problem that silently undermines public safety, weakens critical infrastructure, and costs the global economy more than $2.5 trillion annually. World Corrosion Awareness Day 2025
In this article, we invite you to discover how the phenomenon of environmental corrosion goes far beyond the technical due to its close link with surrounding environmental conditions. It is a problem that transcends the industrial and social spheres and silently infiltrates our daily lives. We also explore the most innovative and accessible solutions on the market, such as environmental monitoring, which not only mitigates its effects but also helps transform our way of coexisting with the environment.

Effects of environmental corrosion on an industrial facility.
What is environmental corrosion and why is it an industrial problem?
Environmental corrosion is an electrochemical phenomenon that causes the gradual degradation of metallic materials and affects most major industrial sectors. It is a process that occurs when reacting with elements present in the environment and generally takes place at ambient temperature through the interaction between corrosive agents in the air (oxygen, water vapour, sulphur dioxide, chlorides, etc.), saline compounds, and industrial pollutants with metallic surfaces.
Air Quality Innovation in Just 1 Click
Stay informed about the air you breathe!
Subscribe to our newsletter to receive the latest updates on environmental monitoring technology, air quality studies, and more.
This process alters the physicochemical properties of metals, compromising both their mechanical strength and functionality. In the industrial context, environmental corrosion represents a critical structural risk, as it can directly impact the operational reliability of equipment, facilities, and systems exposed to harsh environmental conditions.
There is also environmental corrosion by direct contact, which occurs when metals come into physical contact with aqueous solutions, chemicals, or even by reacting with other metals (galvanic corrosion). This generates more localised and faster reactions that can affect specific points of industrial structures.
Environmental corrosion especially impacts the following productive sectors:
- Maritime and port: environments with high exposure to salts and humidity, accelerating the destruction of infrastructure and vessels.
- Industrial and energy: facilities such as chemical plants, refineries, and thermal power plants suffer performance losses and damage to key equipment.
- Cement and biogas plants: the presence of aggressive gases and humidity causes accelerated deterioration of metallic structures and pipelines.
- Aeronautical: aircraft and their components must withstand very aggressive environments with large thermal and humidity fluctuations.
Rapid deterioration in these sectors can drastically reduce the service life of facilities and machinery, compromise safety, and increase the frequency of corrective interventions to repair damage.
The economic costs derived from environmental corrosion include:
- Preventive maintenance and frequent repairs to avoid structural failures or critical breakdowns.
- Production downtime, logistical delays, and reduced operational availability of key infrastructure.
- Structural deterioration requiring early replacement of equipment, constructions, parts, and support systems, impacting investment and profitability.
It is estimated that corrosion represents between 3% and 5% of GDP in highly industrialised countries, given its impacts on safety, productivity, and sustainability. Association for Materials Protection and Performance (AMPP).
To anticipate this environmental problem, the international standard ISO 9223 was developed. This global regulatory standard classifies atmospheres (five categories plus an additional one) according to their corrosive potential on metals exposed to them. It is applied by analysing parameters such as relative humidity, airborne salinity, concentration of pollutants (SO₂, chlorides, etc.), and ambient temperature. In this way, the standard makes it possible to determine the risk of environmental corrosion and define specific protection strategies according to the operating environment. It facilitates the design of intelligent maintenance programmes and the selection of the most appropriate solutions, such as advanced coatings that preserve facilities.

Industrial metallic structure out of use due to environmental corrosion.
Main environmental factors that promote corrosion
The progression of environmental corrosion largely depends on the immediate environmental conditions. Factors such as relative humidity and dew point, temperature and condensation, atmospheric pressure, the presence of pollutant gases, and salinity in coastal areas act synergistically, accelerating or modulating the electrochemical deterioration processes. With Kunak sensors, we can measure this set of parameters in real time and establish direct correlations between their fluctuations and the progression of corrosion, thus anticipating moments of greater risk.
Relative humidity and dew point
Relative humidity determines the thickness of the water film that forms on a metallic surface. When it exceeds the 60–70% level, an electrolyte is created that can dissolve metal ions, accelerating corrosion.
Meanwhile, the dew point indicates the temperature at which that humidity condenses into droplets, promoting the appearance of damp films that act as a conductive medium.
Faced with these environmental fluctuations, Kunak sensors record relative humidity and dew point in real time, allowing to anticipate cycles of water film formation and also quantify their duration. This makes it possible to directly correlate humidity peaks or thermal deficits with increases in corrosion rate.
Temperature and condensation
Temperature is an environmental factor that exponentially influences the kinetics of the electrochemical reactions that cause corrosion. The higher the temperature, the faster the corrosion rate according to the Arrhenius law, but the solubility of gases in surface water is also reduced. Rapid temperature drops in humid environments cause condensation, restarting the corrosive cycle even with brief exposures.
In these situations, Kunak devices continuously measure air and surface temperature, alerting to critical thresholds where sudden condensation raises the rate of corrosive attack. This makes it possible to link thermal rises or falls with specific episodes of accelerated corrosion.
Atmospheric pressure
Pressure variations modify the diffusion of oxygen and atmospheric pollutants to the surface. Lower pressures enhance the adsorption of water vapour and aggressive gases, while higher pressures can force their dissolution through microcracks in metallic surfaces.
The barometric sensor of Kunak AIR stations monitors atmospheric pressure with high resolution, identifying conditions where the diffusion of corrosive agents intensifies. Thus, a direct link is established between pressure fluctuations and variations in the progression of environmental corrosion.
Presence of pollutant gases (SO2, NOx, H2S, O3)
Sulphur and nitrogen oxides, as well as ozone and hydrogen sulphides, react with ambient humidity to form acids that attack the metal. SO2 and NOx generate sulphuric and nitric acid, while O3 acts as a powerful oxidising agent.
Kunak gas detectors capture real-time concentrations of SO2, NOx, H2S, and O3, allowing the plotting of exposure curves and the determination of critical thresholds. This information is key to predicting increases in corrosion rates linked to episodes of high atmospheric pollution.
Salinity in coastal areas
In marine environments, deposited chloride particles are highly hygroscopic, keeping the surface wet even at low relative humidity. This high affinity causes Cl⁻ anions, or chlorides, to directly affect the metal’s passive layer, accelerating cracking and pitting on metallic surfaces.
Kunak stations, measuring in real time both the salinity of the air and the water film on the surface, directly relate salt concentration to the appearance of pitting, enabling real-time monitoring of corrosion progression.

Building structures based on reinforced concrete are among the most affected by environmental corrosion.
How do pollutant gases affect material corrosion?
The presence of pollutant gases in the atmosphere, such as sulphur dioxide (SO2)Sulphur dioxide (SO2) is a colourless gas with a pungent odour that causes an irritating sensation similar to shortness of breath. Its origin is anthropoge...
Read more, nitrogen oxides (NOx) or chlorides in marine environments, significantly accelerates corrosion processes in different metallic materials. When these compounds react with ambient humidity or with a thin film of water on metallic surfaces, they generate highly aggressive agents (for example, SO2 transforms into diluted sulphuric acid). These attack coatings, concrete, and metallic substrates directly—a phenomenon that degrades both the mechanical strength and the aesthetic integrity of the infrastructures to which they belong.
In the case of steel and other metal alloys, exposure to environments loaded with SO2 or NOx causes localised corrosion, the accelerated formation of oxides, and the progressive loss of metal thickness. In reinforced concrete, a double impact is observed: the carbonation of the cement matrix, which reduces the protective pH, followed by the corrosion of internal reinforcement. Even surface protection systems, such as paints and polymer coatings, can suffer chemical degradation, reducing their effectiveness against external agents.
A global transition towards more sustainable, affordable, and reliable energy systems is being driven by the Paris Agreement and the United Nations’ 2030 Agenda for Sustainable Development. This poses a challenge for the corrosion industry, as building climate-resilient energy systems and infrastructure requires a long-term vision, making the long-term behaviour of structural materials (mainly metals and alloys) a key factor. Roman Bender et al. Corrosion challenges towards a sustainable society.
The effects of environmental corrosion are particularly evident in highly exposed sectors such as petrochemical refineries, cement plants, port facilities, urban tunnels with high concentrations of combustion gases, and industrial environments where accelerated corrosion compromises both safety and the durability of installations.
Consequently, the continuous monitoring of atmospheric pollutants and compliance with air qualityAir quality refers to the state of the air we breathe and its composition in terms of pollutants present in the atmosphere. It is considered good when poll...
Read more regulations are essential tools for the protection of strategic infrastructure and the reduction of long-term maintenance costs.

Industrial metallic gear parts affected by environmental corrosion.
Technology to monitor corrosive environments
The supervision of industrial environments with high corrosive potential requires the implementation of cutting-edge technological solutions. These are the means to ensure both asset protection and the maintenance of safe operating conditions.
Which sensors are used?
For precise control in facilities with corrosive atmospheres, gas sensors (H2S, SO2, NOx and other corrosive agents), temperature, humidity and pressure sensors are used, as well as electrical resistance devices, ultrasound, electrochemical and optical sensors. These sensors make it possible to thoroughly assess both environmental conditions and their fluctuations in order to diagnose the progression of corrosion on materials and structures.
Advantages of using modular and smart sensors
The most advanced solutions, such as Kunak AIR modular and smart systems, stand out for their flexibility and scalability at each location. They allow users to select and combine pollutants according to project needs, integrating climatic and chemical variables in a single unit, which remains connected via wireless communications for remote management. This technological architecture facilitates adaptation to diverse industrial facilities, reduces deployment costs, and accelerates operational rollout.
Data analysis and early warning
The distinctive value of monitoring lies in the advanced management enabled by the data obtained from the stations. Specialised platforms such as Kunak AIR Cloud turn data into actionable insights, centralising information, enabling alert algorithms, and providing predictive analytics that prevent structural damage. Real-time access to collected data and early warning features optimise decision-making, maximise asset service life, and lower industrial maintenance costs.

Industrial facilities are heavily affected by environmental corrosion.
Real applications: Where does it make sense to measure air corrosivity?
Assessing air corrosivity is essential in environments exposed to aggressive atmospheres, as these can intensify the deterioration of materials and systems, directly affecting safety, operational performance, and infrastructure durability.
Industrial environments (chemicals, metallurgy, energy)
In specific sectors such as chemicals, metallurgy, and power generation, exposure to corrosive gases, high humidity, and aggressive agents like H2S or SO2 justifies the continuous monitoring of the atmosphere. Corrosion can compromise critical equipment, process lines and instrumentation, causing costly plant shutdowns and reducing the service life of industrial assets.
In a plant dedicated to biogas production, Kunak technology has been implemented to detect the high levels of corrosion affecting certain areas of the facilities. To this end, continuous monitoring is carried out for gases such as hydrogen sulphide (H2S), sulphur dioxide (SO2), methane (CH4)Methane, known chemically as CH₄, is a gas that is harmful to the atmosphere and to living beings because it has a high heat-trapping capacity. For this ...
Read more and nitrogen dioxide (NO2)Nitrogen dioxide (NO2) is a harmful gas whose presence in the atmosphere is mainly due to the use of fossil fuels in combustion vehicles and industrial act...
Read more, in addition to measuring ozone (O3) to correct possible interferences in the detection of NO2. The existence of methane leaks in the system is also being assessed.
So far, significant concentrations of H2S and CH4 have been identified in the environment, probably originating from leaks in the production process. As an initial measure, methane emissions have been addressed, as they pose a risk to both worker safety and the integrity of the facilities, as well as causing significant economic losses for the company.
At the same time, Kunak continues to delve deeper into the analysis of the corrosion processes present at the plant. The high concentrations of methane could be related to the increase in H2S in the surroundings, a highly corrosive gas, possibly due to localised leaks in the digester covers.
Port and coastal infrastructures
For facilities located near the sea—such as ports, platforms, shipyards, or logistics infrastructures—there are high levels of salinity and humidity, which promote the accelerated corrosion of metals and electronic components. Measuring air corrosivity is critical for planning appropriate maintenance activities, protecting industrial structures, and applying preventive coatings.
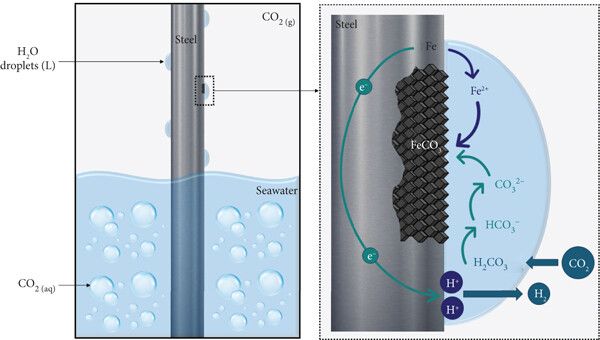
Diagram of environmental corrosion in steel in the presence of saline humidity.
Cities with heavy traffic or urban tunnels
In urban environments with high vehicle density or enclosed spaces such as tunnels, the presence of atmospheric pollutants such as nitrogen oxides, sulphur dioxide, or volatile organic compounds increases the reactivity of the air. This phenomenon accelerates the environmental corrosion of metallic infrastructure, signage, and electrical systems. Continuous air monitoring in these environments enables preventive measures and improved management of urban assets.
Warehouses, stations, and airports exposed to pollutants
Large logistics spaces—such as passenger airport terminals, railway stations, and warehouses exposed to sources of environmental pollution—require strict corrosivity control to preserve electronic systems, control networks, and construction materials. Establishing a continuous diagnostic protocol helps prevent unexpected failures, minimise operational risks, and plan effective maintenance interventions.

Demolition of an industrial plant affected by environmental corrosion.
Standards and recommendations for classifying corrosive atmospheres
The relationship between atmospheric pollution and corrosion is widely recognised in regulations and frameworks that set emission limits for critical compounds. European directives such as 2010/75/EU on industrial emissions, or international standards for classifying corrosive atmospheres (ISO 9223), define the appropriate environmental parameters of limit values and categories to quantify effects and thereby prevent the technical risk of premature and critical degradation of metallic materials and exposed installations.
Among the main regulations are:
- Directive 2010/75/EU, together with its updates and sectoral BREFs, requires industrial facilities to ensure that SO2 and NOx emissions do not exceed the levels associated with Best Available Techniques (BAT).
- The ISO 9223 standard is the main reference defining atmospheric corrosivity categories (C1 to CX) based on the corrosion rate observed in standard specimens after one year of exposure. It assesses the combination of key environmental factors influencing corrosion: temperature, relative humidity, sulphur dioxide (SO2) pollution, and dry deposition of chlorides (airborne salinity) through dose–response functions for different structural metals.
- To verify and confirm these categories, the ISO 9226 standard regulates standardised field tests, detailing exposure methods and analysis of samples exposed to different atmospheric environments to obtain reliable empirical data.
In addition, there are complementary European standards such as:
- EN 13573: focused on corrosion protection and durability of paints and coatings.
- EN ISO 8565 relating to corrosion phenomena and protection of metals and alloys in industrial processes.
Both establish additional technical criteria on protective measures and the assessment of material behaviour in aggressive environments.
In urban and port contexts, the combination of gaseous pollutants and humidity often exceeds the reference values established by ISO 9223 and European directives, requiring monitoring strategies and corrosion-resistant materials to ensure the service life of key infrastructure.
Effective implementation of these standards requires continuous, systematic environmental measurements that allow real-time control of the variables defining air corrosivity. This is key to certifying the assigned corrosivity category, anticipating the impact on assets, and taking preventive decisions on maintenance or design improvements, thus ensuring compliance, safety, and resource optimisation.
In short, accuracy in classifying corrosive atmospheres through these international standards and constant environmental measurements is indispensable for the proper management of anticorrosion protection in industrial and urban environments.

Preventing environmental corrosion in construction is critical to preserving structures.
Advantages of using environmental sensors to prevent corrosion
The use of environmental sensors integrated into monitoring stations to prevent environmental corrosion offers notable benefits for industrial environments and infrastructure. These devices enable real-time monitoring of critical parameters such as humidity, temperature, and pollutant concentration, providing reliable data to anticipate risks before they become serious and costly repair or maintenance issues.
With precise information on environmental conditions, it is possible to optimise the planning of inspections and maintenance operations, avoiding unexpected corrective interventions that raise overall costs. In addition, continuous monitoring extends asset service life by allowing the detection of aggressive environments and the implementation of timely preventive measures. In this way, sensors provide an objective basis for technical decision-making, enabling more effective management strategies grounded in real evidence.

Ship anchor chain affected by environmental corrosion intensified by saline environments.
Frequently asked questions about environmental corrosion
What is atmospheric corrosion and how is it measured?
Atmospheric corrosion is a phenomenon of chemical or electrochemical degradation that mainly affects metals when they come into contact with elements present in the air. This process develops according to specific environmental conditions, including relative humidity, temperature, time of wetness, and the concentration of aggressive agents and pollutants in the air.
To characterise and classify the aggressiveness of the environment, international standards are used such as ISO 9223, which establishes atmospheric corrosivity categories, and ISO 8565, which defines exposure methods for field tests.
Which gases most aggravate material corrosion?
The air pollutantsAir pollution caused by atmospheric contaminants is one of the most critical and complex environmental problems we face today, both because of its global r...
Read more that most intensify the corrosion of materials are sulphur dioxide (SO2), nitrogen oxides (NOx), and chlorides from the marine environment. The presence of these gases—especially when coexisting with humidity—accelerates material reactions. Water acts as a dissolution and reaction medium, facilitating the formation of strong acids (such as sulphuric or nitric acid), which attack the surface of materials—paints, metals, and reinforced concrete—causing localised corrosion, coating degradation and, in the case of concrete, damage to structural integrity.
Can corrosion be predicted with sensors?
Yes, environmental corrosion can be predicted thanks to smart sensors integrated into real-time air monitoring stations that collect data on environmental and physicochemical parameters. These devices record data such as concentrations of air pollutants, humidity, temperature, and salt deposition, enabling trend estimation and early risk prediction for infrastructure exposed to corrosion.
Which industries need to control environmental corrosion?
Environmental corrosion is a critical challenge for multiple industries, especially those whose infrastructure is exposed to aggressive or polluted atmospheric conditions. The most vulnerable sectors include:
- Refineries and petrochemical complexes: where the presence of sulphur compounds and humid environments promotes accelerated corrosion of metallic equipment.
- Cement and reinforced concrete industries: exposed to acidic gases and particles that compromise the structural integrity of their facilities.
- Port and maritime infrastructure: subject to saline environments and high humidity—ideal conditions for chloride-induced corrosion.
- Urban tunnels, high-traffic areas and rail systems: where condensation, pollution and poor ventilation can create highly corrosive environments.
- Power generation plants and water treatment stations: operating with metallic equipment sensitive to corrosion from chemical agents and constant humidity.
Preventive management of corrosion in these sectors not only extends asset service life, but also:
- Reduces operating costs associated with repairs and replacements.
- Minimises the risk of structural failures that could compromise safety.
- Optimises operational efficiency by avoiding unplanned shutdowns.
In short, implementing air monitoring strategies, selecting corrosion-resistant materials, and applying predictive maintenance are essential to ensure the long-term sustainability and reliability of these industries.
What is the difference between corrosion sensors and environmental sensors?

Metal propeller affected by environmental corrosion.
Environmental sensors measure general variables such as temperature, relative humidity, and pollutant concentrations, providing a global view of the assessed environment. In contrast, corrosion sensors (mass loss, electrical resistance or electrochemical techniques) are designed to quantify the deterioration process in specific materials, allowing direct evaluation of the level or rate of atmospheric attack on a structure. In an advanced air-monitoring strategy, both types of sensors are combined to provide predictive and tailored management of industrial corrosion.
Conclusion – Towards proactive corrosion management
Air quality is not only an environmental challenge; it also acts as a critical factor in the durability and reliability of infrastructure in industrial settings. The presence of corrosive gases such as SO2, NOx and chlorides, together with ambient humidity, increases the vulnerability of structural materials and demands a preventive approach based on continuous monitoring and regulatory compliance.
Having smart sensors capable of measuring, recording and anticipating variations in the environmental and physical parameters of assets enables industrial operators to shift towards the predictive management of corrosion. The strategic deployment of these devices makes it possible to detect deterioration trends before they turn into costly structural failures, optimise maintenance resources and reinforce industrial safety, thereby adding value in a responsible and sustainable manner.
Adopting digitalised control platforms not only meets the requirements of global regulations, but also promotes a proactive and thoughtful business approach to protecting critical assets. The combination of real-time data and predictive modelling is now the most effective tool to anticipate risks caused by atmospheric pollution and ensure the service life of industrial facilities.
References
- Saltos Montaño, J.P. and Torres Calle, M.L. (2010). Characterisation of the most important atmospheric pollution parameters for atmospheric corrosion in the cities of Esmeraldas and Santo Domingo de los Colorados. Part I. Digital Repository – EPN, Faculty of Chemical Engineering and Agro-Industry (FIQA), Chemical Engineering (IQUIM), Chemical Engineering Thesis (IQUIM). https://bibdigital.epn.edu.ec/handle/15000/2642
- Bender, R. et al. (2022). Corrosion challenges towards a sustainable society. Materials and Corrosion, Volume 73, Issue 11, November 2022, pp. 1730–1751. https://doi.org/10.1002/maco.202213140
- Prasad A., Kunyankandy A., Joseph A. (2020) Corrosion Inhibition in Oil and Gas Industry. Editors: Viswanathan S. Saji, Saviour A. Umoren. https://doi.org/10.1002/9783527822140.ch5
- Klodian Xhanari, Yefei Wang, Zhen Yang, Matjaž Finšgar. (2021). A Review of Recent Advances in the Inhibition of Sweet Corrosion. The Chemical Record, Volume 21, Issue 7, July 2021, pp. 1845–1875. https://doi.org/10.1002/tcr.202100072
- Fonseca D., Tagliari M. R., Guaglianoni W. C., Tamborim S. M., Borges M. F. (2024). Carbon Dioxide Corrosion Mechanisms: Historical Development and Key Parameters of CO2-H2O Systems. International Journal of Corrosion. Volume 2024, Issue 1. Jan 2024. https://doi.org/10.1155/2024/5537767









