What is hydrogen cyanide (HCN)
It would be impossible to enjoy ubiquitous plastics, the control function of pesticides, or extract precious metals without hydrogen cyanide (HCN). A colourless and highly volatile gas, as toxic as it is essential for industry. The extreme hazard of handling this chemical lies in its ability to block cellular respiration; even dermal absorption at high concentrations can cause death within minutes of exposure.
Although highly toxic, recent studies by the CSIC have shown it may have regulatory effects on plant development, potentially opening up new industrial and agricultural applications.
The toxicity depends on whether cyanide is in free form (gas or liquid) or complexed (aqueous or solid). It can be inhaled (gas), ingested (liquid or solid), or absorbed through the skin. Acute poisoning in humans leads to seizures, vomiting, coma, and death. Guerrero, J., 2015. Cyanide: Toxicity and Biological Destruction.
The purpose of this article is to address the sources of hydrogen cyanide, the industrial risks associated with its use, its impact on health and the natural environment, and to highlight the most modern technological tools for its monitoring. These make it possible to manage HCN safely, complying with strict industrial controls designed to prevent accidents, protecting both workers and the environment, thanks to established occupational exposure limits.
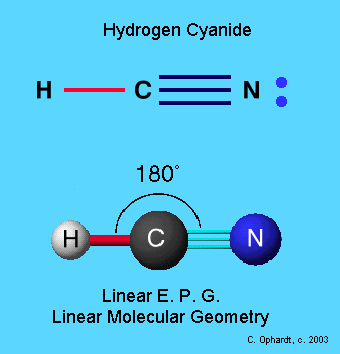
Composition of the hydrogen cyanide molecule
Chemical properties and uses of HCN
HCN, also known as hydrocyanic acid, is a volatile chemical compound with a relatively low boiling point of 26 °C. It appears as a colourless liquid at low temperatures, becoming gaseous at room temperature. It has a characteristic bitter almond odour, perceptible to many but not to those with a recessive genetic trait (20–40% of the population) that prevents detection. This implies an additional risk, which is why in industrial settings the presence of HCN should not be detected by smell but by advanced monitoring technologies to prevent poisoning and accidents.
Molecular structure and physicochemical characteristics
Hydrogen cyanide (HCN) is a linear molecule composed of one hydrogen, one carbon, and one nitrogen atom (H–C≡N), with a triple bond between carbon and nitrogen and a single bond between hydrogen and carbon. The carbon hybridisation is sp, explaining its linear geometry and H–C–N bond angle of 180°. Despite its linearity, the molecule is not neutral but polar, due to electronegativity differences between hydrogen, carbon, and nitrogen, creating dipole moments or charge separation within the molecule. This influences many of its chemical and physical properties, such as solubility, reactivity, and behaviour under electric fields.
Air Quality Innovation in Just 1 Click
Stay informed about the air you breathe!
Subscribe to our newsletter to receive the latest updates on environmental monitoring technology, air quality studies, and more.

The plastics industry relies on hydrogen cyanide as an essential raw material.
Main industrial applications (plastics, mining, organic synthesis)
Hydrogen cyanide is crucial across several industries essential to society. In the plastics industry, it is the basic raw material for the synthesis of acrylonitrile, used in the manufacture of synthetic fibres, resins, and a wide range of plastics. In mining, HCN plays a key role in the leaching of gold and silver, fundamental processes for efficient extraction of these precious metals. It is also essential in organic and fine chemistry, producing various compounds and intermediates including pesticides, pharmaceuticals, and other industrial materials.
Regulations and occupational exposure limits for HCN
Due to its high toxicity, HCN exposure is strictly regulated at international and national levels. Generally, regulations establish very low limits for tolerating the presence of this gas in workplaces; maximum permissible concentrations are around 10 ppm (parts per million) over a working day to avoid acute and chronic effects in exposed individuals.
Laws require stringent environmental monitoring and control measures for the early detection and mitigation of HCN in industrial facilities, including the use of personal protective equipment and specialised ventilation systems, ensuring worker safety and minimising environmental risks. These regulations are constantly updated to reflect advances in toxicology and control technologies.

Gold extraction requires the use of hydrogen cyanide.
Sources of emission and detection of HCN in air
Cyanides, a diverse family of compounds containing the highly reactive cyanide anion (CN–), are produced from both anthropogenic and natural sources. Agency for Toxic Substances and Disease Registry. (2019). Toxicological Profile for Cyanide. U.S. Department of Health and Human Services, Public Health Service.
Chemicals that release cyanide are called cyanogenic compounds. The most common cyanides in the environment are sodium cyanide, potassium cyanide, and gaseous hydrogen cyanide, the latter being the main form present in air.
HCN is a highly volatile chemical with high atmospheric dispersion capacity and extreme toxicity. Its release into the environment is mainly linked to industrial processes and combustion. Identifying its emission sources is crucial to design mitigation and control strategies that reduce risks, protecting public health and the natural environment.
Production processes of cyanides and combustion
One of the main pathways of HCN generation is linked to the synthesis and transformation of cyanide salts, widely used in the chemical industry for producing plastics, synthetic fibres, and pesticides. During these operations, HCN may be released as a gaseous by-product if proper containment and scrubbing systems are not in place. Likewise, the incomplete combustion of nitrogen- and carbon-containing materials (such as plastics, polyurethanes, or even biomass) is another major source: under high-heat but oxygen-deficient conditions, thermochemical reactions promote the formation of HCN precursor radicals, directly affecting exposed workers.
In the incomplete combustion of nitrogenous polymers such as polyurethane foams during fires, elevated HCN peaks are observed in air. This makes HCN a critical factor in fire smoke toxicity, increasing both domestic fire mortality and the risk for firefighters and rescue teams.
Fugitive emissions, accidental releases, and exposure
In addition to controlled chronic emissions, even in small amounts that degrade air qualityAir quality refers to the state of the air we breathe and its composition in terms of pollutants present in the atmosphere. It is considered good when poll...
Read more in enclosed workplaces, there are also unintentional losses or fugitive emissions. These include leaks in valves, pipe joints, or storage systems.
In facilities handling cyanides, minor seal failures may lead to continuous low-intensity releases, still potentially dangerous. Added to this are larger-scale incidents, such as spills or leaks during loading and unloading, where contact with acids or improper pH conditions can suddenly trigger a gaseous release of HCN, affecting nearby communities and aquatic fauna.
High-risk sectors: metallurgy, galvanisation, and refining
Certain sectors such as extractive metallurgy have a greater propensity to generate or release HCN. Extractive metallurgy, using cyanidation in the recovery of metals such as gold and silver, is one of the most critical activities, as it generates health risks for workers and receiving water bodies if effluents are not properly controlled.
Galvanisation and other metal surface treatments also involve cyanide baths, where operating conditions are critical to prevent emissions and serious inhalation risks in workshops. In the refining industry, both metals and petroleum, secondary or diffuse releases may occur from waste combustion and high-temperature operations, affecting not only local air quality but also nearby communities.

Pesticides used in agriculture require hydrogen cyanide for their production.
Effects of HCN on health, the environment, and occupational safety
Hydrogen cyanide and its cyanide derivatives pose risks to human health, the natural environment, and the safety of workers in high-risk industrial sectors. Its acute toxicity, the consequences of prolonged exposure, and its effects on ecosystems require comprehensive control and management.
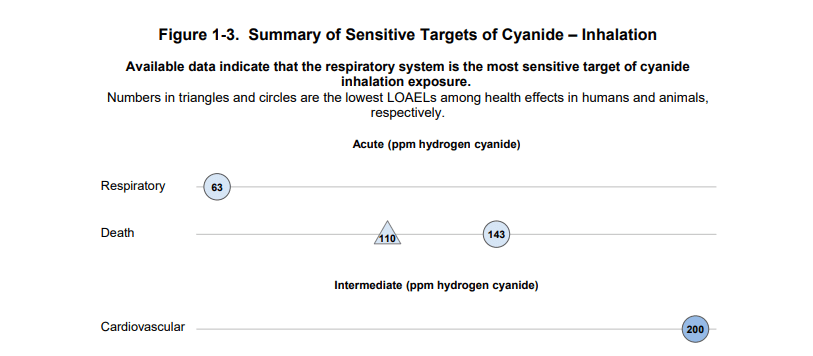
Targets of cyanide inhalation exposure in humans.
Acute and chronic toxicity in humans
HCN is a lethal gas even at relatively low concentrations due to its ability to inhibit the mitochondrial enzyme cytochrome c oxidase. This mechanism means that cells cannot use available oxygen (histotoxic anoxia), rapidly causing cardiac and neurological dysfunction.
In acute exposures, early symptoms include headache, dizziness, dyspnoea, and tachycardia, potentially progressing quickly to seizures, loss of consciousness, and cardiorespiratory arrest.
In chronic exposures, even at low doses, there are persistent effects on the central nervous system (cognitive disorders, recurrent headaches) and thyroid alterations, as cyanides interfere with iodine metabolism. Workers routinely exposed in processes involving cyanides also experience mucosal irritation and skin disorders.
“Hydrogen cyanide is a systemic chemical asphyxiant. It interferes with the normal use of oxygen by nearly all organs of the body. Exposure to hydrogen cyanide can be immediately fatal. It has systemic effects throughout the body, particularly on systems most sensitive to low oxygen levels: the central nervous system (brain), the cardiovascular system (heart and blood vessels), and the respiratory system (lungs).” The National Institute for Occupational Safety and Health (NIOSH)
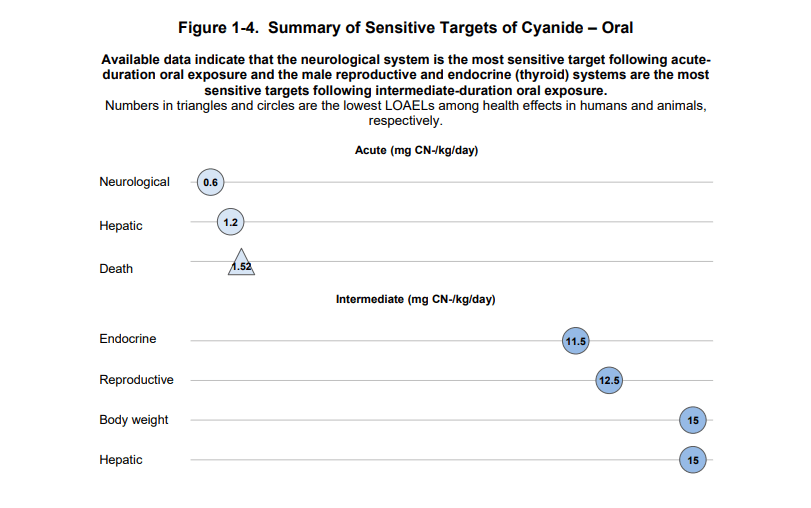
Human systems sensitive to oral cyanide exposure.
Respiratory and neurological effects of HCN
Respiratory damage is among the most severe manifestations of HCN exposure, ranging from bronchial irritation to pulmonary oedema under significant exposures. Neurological impairment ranges from mild symptoms, such as anxiety and tremors, to toxic encephalopathy in cases of prolonged exposure. These effects are particularly concerning in occupational settings, where sudden accidental exposure can cause immediate incapacitation—compromising not only individual health but also industrial operational safety.

Application of pesticide in an agricultural crop.
Environmental impact: soils, waters, and wildlife
HCN released into the atmosphere disperses and can deposit onto soils and water bodies as cyanide ions. In terrestrial environments, these compounds reduce soil fertility by altering microbial activity essential for nutrient cycling. In natural waters, cyanides are highly toxic to fish and aquatic invertebrates by blocking cellular respiration; even concentrations of a few milligrams per litre can cause mass mortality.
In terrestrial ecosystems, mammals and birds exposed to contaminated water or food may show signs of acute poisoning, thereby compromising local biodiversity and food webs.
Documented cases of industrial exposures
Incidents have been recorded at galvanising plants, refineries, and metal processing facilities where uncontrolled leaks or reactions released HCN. Documented cases show recurring scenarios: multiple poisonings among maintenance staff due to inadequate ventilation, communities affected by accidental emissions, and mortality among aquatic fauna after non-neutralised discharges. These events have led to the inclusion of HCN in regulations on extremely hazardous substances, reinforcing the need for contingency plans, environmental monitoring, and rapid-response drills.
Why is it necessary to monitor HCN?
Techniques, HCN sensors, and detection technologies
Monitoring HCN is essential due to its high toxicity, volatility, and dispersion in workplace and industrial environments. Prolonged exposure, even at low concentrations, poses a risk to workers’ health and safety, as well as a potential environmental impact. Therefore, integrating accurate and reliable detection techniques is crucial both for accident prevention and for regulatory compliance.
Detection limits and regulatory thresholds
Hydrogen cyanide (HCN) is an extremely toxic gas; for this reason, various international bodies have set very strict safety limits to protect workers’ health.
- In the United States, for example, the OSHA (Occupational Safety and Health Administration) permits a maximum exposure (PEL, Permissible Exposure Limit) of 10 ppm over an eight-hour working shift. Other bodies are even more rigorous: the ACGIH (American Conference of Governmental Industrial Hygienists) recommends never exceeding 4.7 ppm, and NIOSH (The National Institute for Occupational Safety and Health) aligns with this value, adding that a concentration of 50 ppm is already immediately dangerous to life.
- In Europe, the Directive 2000/39/EC sets similar reference values, which each country adapts to its national regulations. In Spain, for example, the INSST reflects these limits in its occupational exposure tables.
In short, we are dealing with very narrow margins: minute amounts of HCN can pose a serious risk. Therefore, monitoring must be highly precise, capable of detecting the gas not only at ppm levels in workplace environments, but even at ppb in ambient air. Only then can we ensure the early detection of leaks and protection against exposure, both acute and prolonged.
Electrochemical sensors vs gas chromatography for HCN
The two main HCN detection techniques serve different needs:
Electrochemical sensors
Monitoring with electrochemical sensors is vital in industrial environments thanks to their rapid response, compact size, and portability, enabling real-time detection and personal protection. In Kunak monitoring equipment, the HCN cartridge includes an integrated electrochemical sensor capable of detecting low HCN concentrations, from below 1 ppm up to 50 ppm, with an accuracy of ±0.10 ppm. This sensor is ideal for continuous surveillance in industrial applications where hydrogen cyanide control is critical.
However, it should be noted that it exhibits significant cross-sensitivity to gases such as SO2, NO2 and CO. Therefore, to ensure maximum accuracy in environments with elevated levels of these gases, it is recommended to use NO2, O3, SO2 and CO cartridges alongside the HCN cartridge. This allows Kunak’s algorithm to compensate for these interferences and deliver more reliable, accurate measurements.
Gas chromatography (GC)
A reference technique in laboratories and for environmental control. It enables ultraselective detection and quantitative analysis of HCN at trace levels, even in complex mixtures. Combined with specific detectors (FID, MS or TCD), it offers high sensitivity and analytical robustness. However, due to cost and the need for qualified personnel, it is generally used for validations, environmental studies, and forensic applications rather than continuous monitoring.
Fixed AAQMS networks and continuous HCN monitoring
In high-risk locations such as chemical plants, metallurgical industries, or urban areas near emission sources, fixed monitoring networks connected to AAQMS systems (Ambient Air Quality MonitoringControlling air quality is an essential task in order to enjoy optimal environmental conditions for healthy human development and to keep the environment i...
Read more Systems) are implemented. These networks enable:
- Real-time surveillance with continuous data transmission.
- Integration of different sensors (electrochemical, optical, spectroscopic) in multi-parameter systems.
- Activation of emergency protocols when safety thresholds are exceeded.
- Added value through temporal and spatial correlation, offering a dynamic view of pollutant dispersion in the environment.
Data management platforms, early warnings, and HCN-in-air analytics
The final link in a modern HCN monitoring strategy is intelligent data management. Via cloud platforms and SCADA (Supervisory Control and Data Acquisition) systems, field data are processed in real time to:
- Establish trends and detect deviations.
- Implement predictive dispersion models based on machine-learning algorithms.
- Send automatic notifications and early warnings to safety managers and exposed populations.
In this way, not only is immediate response capability improved, but it also contributes to the optimisation of industrial processes and the demonstration of regulatory compliance during inspections and audits.
FAQs about HCN
What is the maximum permitted HCN exposure level?
The maximum permitted exposure level for hydrogen cyanide (HCN) varies by international regulation, but the most recognised are:
- OSHA (Occupational Safety and Health Administration, US): sets a Permissible Exposure Limit (PEL) of 10 ppm as an average over an 8-hour work shift.
- NIOSH (The National Institute for Occupational Safety and Health, US): recommends a short-term exposure limit of 7 ppm (5 mg/m³) for a maximum of 15 minutes, which should never be exceeded.
- ACGIH (American Conference of Governmental Industrial Hygienists): likewise sets a Short-Term Exposure Limit (STEL) of 7 ppm, with the same restriction that it must not be exceeded at any time.
- In Europe, Directive 2000/39/EC establishes similar values, recognising the immediately dangerous nature of this gas.
In addition, the Immediately Dangerous to Life or Health (IDLH) level is set at 50 ppm, the threshold above which exposure can cause immediate fatal harm and requires self-contained respiratory protection for workers.
How can HCN leaks be detected in real time?
Real-time detection of hydrogen cyanide (HCN) leaks is primarily based on the use of electrochemical sensors integrated into continuous monitoring systems. This is the most widely adopted technology due to its accuracy, speed, and ease of integration into portable and fixed systems.
These sensors operate via an electrochemical reaction: HCN in the air reacts at the electrode surface, generating an electrical current proportional to its concentration. This signal is processed immediately to indicate the presence and level of the toxic gas, enabling rapid action. The key advantages of electrochemical HCN sensors are their low detection limit, ability to provide accurate measurements at very low concentrations, and a very short response time, ideal for early leak alerts.
What equipment and PPE should workers exposed to HCN use?
Workers handling hydrogen cyanide (HCN) must be equipped with specialised protective elements to prevent exposure to this extremely toxic gas, which can be fatal within minutes—or even seconds—if a certain concentration threshold is reached. The minimum essential equipment includes:
- Portable HCN detector: regulations require workers to carry an individual portable gas detector specifically calibrated for HCN. This enables rapid identification of dangerous concentrations and immediate action.
- Self-contained breathing apparatus: in areas with a permanent risk of HCN presence, it is mandatory to have and potentially use SCBA when the detector indicates dangerous concentrations or there is a possibility of exceeding them.
- Appropriate respiratory protection: for handling and confirmed presence of HCN, a full-face mask with filters for acidic gases and cyanides (e.g., type B2/3), preferably combined with a P3 particulate filter, must be used. In high-risk or prolonged exposure scenarios, air-supplied equipment is essential.
- Eye protection: sealed goggles or safety eyewear certified to EN 166 to prevent splashes or eye contact.
- Hand protection: long neoprene or butyl gloves specifically designed to resist HCN permeation and its compounds, preventing dermal contact.
- Body protection: chemical protective clothing (impermeable coverall, rubber or PVC boots). If exposure is potentially high or prolonged, fully impermeable garments are recommended to minimise skin contamination.
- Additional conditions: access should be limited to essential personnel and for the shortest possible time. Risk areas must be demarcated and signposted, and workers should always be accompanied during interventions. Protective equipment must be kept in perfect condition and checked before and after each use.
How is an accidental HCN release neutralised?
In the event of an accidental release of hydrogen cyanide (HCN), a rapid and safe neutralisation must be carried out by strictly following technical emergency protocols to prevent poisoning and environmental dispersion. The process includes the following essential steps:
- Evacuation and area control: evacuate immediately and restrict access to protected personnel only. Place barriers or dykes to prevent the spill from reaching water bodies or the sewer system due to its high toxicity and explosion risk.
- Ventilation: allow the gas to disperse naturally. If the leak comes from a cylinder and cannot be sealed, move it to an open, safe area until it is emptied or repaired. Do not use forced ventilation that could spread the gas.
- Containment: cover liquids with inert absorbents (sand, earth) and store them in airtight containers for hazardous waste management. Do not use water. Cover with plastic sheeting to prevent volatilisation.
- Neutralisation: apply oxidising solutions (5% hypochlorite or peroxide) while maintaining pH > 10 to transform cyanide into less toxic compounds and avoid the formation of HCN.
- Waste: treat as hazardous waste in accordance with regulations; never discharge into water bodies or the sewer system.
- Equipment: use non-sparking, grounded containers and tools, as HCN is flammable.
Consequently, effective neutralisation relies on a combination of efficient evacuation, physical containment, safe ventilation, and oxidative chemical treatment with suitable solutions—always under specialised protection and in line with strict regulatory protocols.
What are the long-term impacts of low HCN exposure?
Chronic exposure to low concentrations of hydrogen cyanide (HCN) can cause a range of long-term adverse effects. It primarily affects the nervous system, thyroid function, and the blood. Technical and epidemiological effects include:
- Respiratory and cardiac conditions: workers exposed to concentrations as low as 6–10 ppm for years have experienced breathlessness, chest pain, vomiting, blood abnormalities, and recurrent headaches.
- Thyroid impact: thyroid enlargement (goitre) and functional changes are common due to inhibition of iodine uptake, especially among workers chronically exposed in electroplating or mining.
- Neurological effects: alterations of the central nervous system, such as weakness, sensory disorders (smell, taste), memory deficits, chronic fatigue, vertigo, balance issues, cognitive difficulties, and—in more severe cases—persistent brain damage following higher exposures.
- Irritation and skin damage: continuous contact can cause dermatitis, rashes, and lesions to skin and mucous membranes, including bleeding and nasal injury in cases of inhalational exposure.
- Haematological alterations: increases in haemoglobin and lymphocytes have been detected due to functional changes in the blood.
In conclusion, chronic, sustained low-dose HCN exposure can cause multisystem symptoms (neurological, thyroid, respiratory, and dermatological) that must be prevented through environmental and medical surveillance of at-risk workers.
Conclusion
Monitoring hydrogen cyanide (HCN) is not optional; it is an essential component of any industrial and environmental risk management strategy. Even very short exposures at low concentrations can have serious consequences for human health and industrial operational safety, as well as causing irreversible environmental impacts. This is compounded by an increasingly stringent regulatory framework requiring reliable detection at ppm and even ppb levels.
In this context, proactive monitoring offers tangible benefits:
- Prevention of accidents and reduction of occupational exposure.
- Compliance with international regulatory limits.
- Greater response capability to incidents thanks to early warning systems.
- Process optimisation and cost reduction associated with unplanned shutdowns or regulatory penalties.
Current technology based on high-precision sensors, continuous monitoring networks, and intelligent data management platforms not only allows real-time detection of HCN leaks or hazardous concentrations, but also anticipates risk scenarios through predictive analytics.
Within this framework, Kunak solutions are positioned as a key ally, offering an end-to-end tool for early detection and efficient management of HCN. Adopting these technologies is not only about meeting regulatory requirements; it also moves organisations towards a preventive model in which the safety of people and workers, alongside environmental sustainability, is central to any industrial operation.
Ultimately, investing in intelligent HCN monitoring is not merely a matter of compliance; it is a strategic decision that protects lives, ensures the continuity of industrial processes, and strengthens environmental responsibility across the industry.
References
- Agency for Toxic Substances and Disease Registry. (2019). Toxicological Profile for Cyanide. U.S. Department of Health and Human Services, Public Health Service. Retrieved from https://www.atsdr.cdc.gov/ToxProfiles/tp8.pdf
- World Health Organization. (2003). Hydrogen cyanide and cyanide salts: Environmental Health Criteria 47. Geneva: WHO. https://apps.who.int/iris/handle/10665/42717
- National Institute for Occupational Safety and Health. (2021). NIOSH Pocket Guide to Chemical Hazards: Hydrogen Cyanide. Centers for Disease Control and Prevention. Retrieved from https://www.cdc.gov/niosh/npg/npgd0317.html
- Ni, Y., Zheng, S., Li, X., & Wang, H. (2018). An amperometric electrochemical sensor for hydrogen cyanide detection in industrial environments. Sensors and Actuators B: Chemical, 255, 1450–1457. https://doi.org/10.1016/j.snb.2017.08.087
- Zhang, L., Martínez, A., & Pérez, J. (2020). Real-time monitoring of hydrogen cyanide emissions in chemical plants using portable sensor arrays. Journal of Environmental Chemical Engineering, 8(5), 104239. https://doi.org/10.1016/j.jece.2020.104239




