The carbon monoxide (CO) is an invisible gas (colorless and odorless) that, at the same time, is a silent killer because in just a few minutes it exhibits its dangerous toxic effect when inhaled in places with large amounts of gas.
It primarily originates from incomplete combustion (due to lack of oxygen) of fuels such as gasoline, gas, coal, oil, wood, and petroleum. Therefore, it is our daily activities, like the operation of vehicle engines, heating systems, ovens, fireplaces, generators, and any other apparatus that burns fossil fuels which expose us to the effects of this toxic gas present in the air.
Additionally, it is a highly flammable gas that reacts violently upon contact with oxygen, nitrogen oxides, chlorine, fluorine, and acetylene, among other substances. This leads to the production of toxic fumes; as well as fires, in the event there are heat sources in contact with CO.

Sources of Carbon Monoxide – CO Emission of CO into the atmosphere – Infographic US-EPA
Air Quality Innovation in Just 1 Click
Stay informed about the air you breathe!
Subscribe to our newsletter to receive the latest updates on environmental monitoring technology, air quality studies, and more.
Main characteristics of carbon monoxide
Without specific devices for its detection, such as air qualityAir quality refers to the state of the air we breathe and its composition in terms of pollutants present in the atmosphere. It is considered good when poll...
Read more sensors, carbon monoxide is a very dangerous gas that surrounds us and threatens health; due to its characteristics (colorless and odorless) that make it imperceptible.
It is a pollutant that, in turn, persists in the air, causing a deterioration of its quality by altering healthy environmental conditions, mainly in urban environments, where it acts as a precursor to photochemical smogSmog, beyond that dense fog
Smog is a mixture of air pollutantsAir pollution caused by atmospheric contaminants is one of the most critical and complex environmental problems we face today, both because of its global r...
Read more that accumulate in the atmosphere, especially in urban areas. This phenomenon is character...
Read more.
Why is carbon monoxide dangerous to health?
The ability of carbon monoxide to bind to the hemoglobin molecule, present in the blood, more easily than the oxygen it usually carries, reduces hemoglobin’s function to adequately oxygenate organs and tissues. A harmful union that results in carboxyhemoglobin, a molecule whose presence in living beings is toxic as it can lead to asphyxiation, brain damage, neurological, cardiac issues or death. And, in prolonged exposures, it causes damage to the nervous and cardiovascular systems.
What are the symptoms of carbon monoxide poisoning?
The main effects caused by breathing in environments with a high concentration of carbon monoxide are dizziness, headache, nausea and vomiting sensations, fatigue, and mental confusion. In more severe cases, it causes a loss of consciousness.
If one perceives these symptoms due to being in an environment with a high concentration of CO, it is vital to leave the area as quickly as possible and notify emergency services or specialized technical teams to intervene in these situations. It is also advisable to not return to the location until the contaminating emissions have been identified and reduced to acceptable levels for breathing without health risks.
How does carbon monoxide affect ecosystems?
The presence of CO in elevated levels reduces the amount of available oxygen in the air, thereby altering aerobic respiration in living organisms; affecting biodiversity in areas where air pollution persists due to the presence of this gas.
Carbon monoxide affects biodiversity, habitats, and ecosystems in an indirect but significant manner, because it alters atmospheric composition. It does so by interacting with other air pollutants, such as methane and ozone, thus aggravating air pollution.
In aquatic ecosystems near CO emission sources, although it is not a gas that dissolves easily in water, when industrial spills increase its concentration in the water, it can cause a lack of oxygen favoring hypoxia or low oxygen levels available for fish species.
In summary, although carbon monoxide does not always act directly, its indirect effects have serious repercussions on ecosystems and their biodiversity. The influence of CO on air quality causes environmental balance and trophic dynamics alterations, underscoring the need to monitor and control air to mitigate these impacts and protect the environment.
How does carbon monoxide contribute to climate change?
Although CO cannot trap heat from the atmosphere directly, it does have a negative impact through the amplifying effects it provokes that accelerate global warming. These interactions place it among the essential pollutants to control to mitigate the effects of climate change.
Carbon monoxide acts by decreasing hydroxyl radicals (OH) present in the air. By doing so, they eliminate the key role these molecules play in the atmosphere because they are molecules that break down potent greenhouse gasesGreenhouse gases (GHGs) are natural and anthropogenic gases that trap heat in the Earth's atmosphere, regulating the planet’s temperature. However, when ...
Read more such as methane. By reducing the availability of OH radicals, it causes methane to remain longer in the atmosphere, triggering its harmful greenhouse effect that accelerates global warming.
The presence of CO in the air also contributes to the formation of fine suspended particles (PM2.5). Similarly, it is a precursor to tropospheric ozone because it promotes the chemical reaction for its formation. It occurs when CO reacts with other atmospheric pollutants, such as nitrogen oxides (NOx) and volatile organic compounds (VOC)Volatile Organic Compounds (VOCs) are chemical substances primarily composed of carbon and hydrogen, but they can also contain other elements such as oxyge...
Read more. Activated under sunlight, they promote the formation of tropospheric ozone, a gas that traps atmospheric heat directly impacting climate change.
In turn, the presence of tropospheric ozone decreases the capacity of vegetation to perform photosynthesis, a fundamental process for capturing much of the carbon dioxide (the main greenhouse gas) present in the atmosphere, thereby altering its important role as a carbon sink.
CO, by contributing to the formation of tropospheric ozone and prolonging the presence of methane in the atmosphere, fosters extreme climatic events (prolonged droughts and forest fires) by increasing global temperatures.
What is the difference between CO and CO2?
While CO and CO2 are gases composed of carbon and oxygen, they differ significantly in structure, properties, and potential effects.
Chemical Composition
- Carbon monoxide (CO): Composed of one carbon atom and one oxygen atom.
- Carbon dioxide (CO2): Composed of one carbon atom and two oxygen atoms.
Formation
- Carbon monoxide: Primarily generated from the incomplete combustion of materials containing carbon, such as gasoline, wood, or natural gas. This occurs when there is little oxygen available during combustion.
- Carbon dioxide: Forms during the complete combustion of materials containing carbon in the presence of sufficient oxygen. It is also produced during biological processes such as cellular respiration and fermentation.
Chemical Properties
- Carbon monoxide: It is a highly toxic, colorless, odorless, and tasteless gas.
- Carbon dioxide: If present in normal concentrations (around 0.04% in the air), it is not toxic. It is colorless and, in high concentrations, has a slightly acidic taste.
Impact on Health
- Carbon monoxide: Extremely dangerous because it can cause anything from poisoning to asphyxiation without the person noticing. Even in small concentrations, it can be lethal if exposure is prolonged.
- Carbon dioxide: Harmless in normal concentrations, however, in high concentrations (e.g., in enclosed or poorly ventilated spaces), it can cause asphyxiation by displacing oxygen.
Environmental Impact
- Carbon monoxide: Develops limited impact as a greenhouse gas but contributes to increasing air pollution and the presence of harmful gases such as methane that contribute to the greenhouse effect.
- Carbon dioxide: It is the main greenhouse gas responsible for global warming. It is released into the atmosphere in large quantities by human activities such as burning fossil fuels.
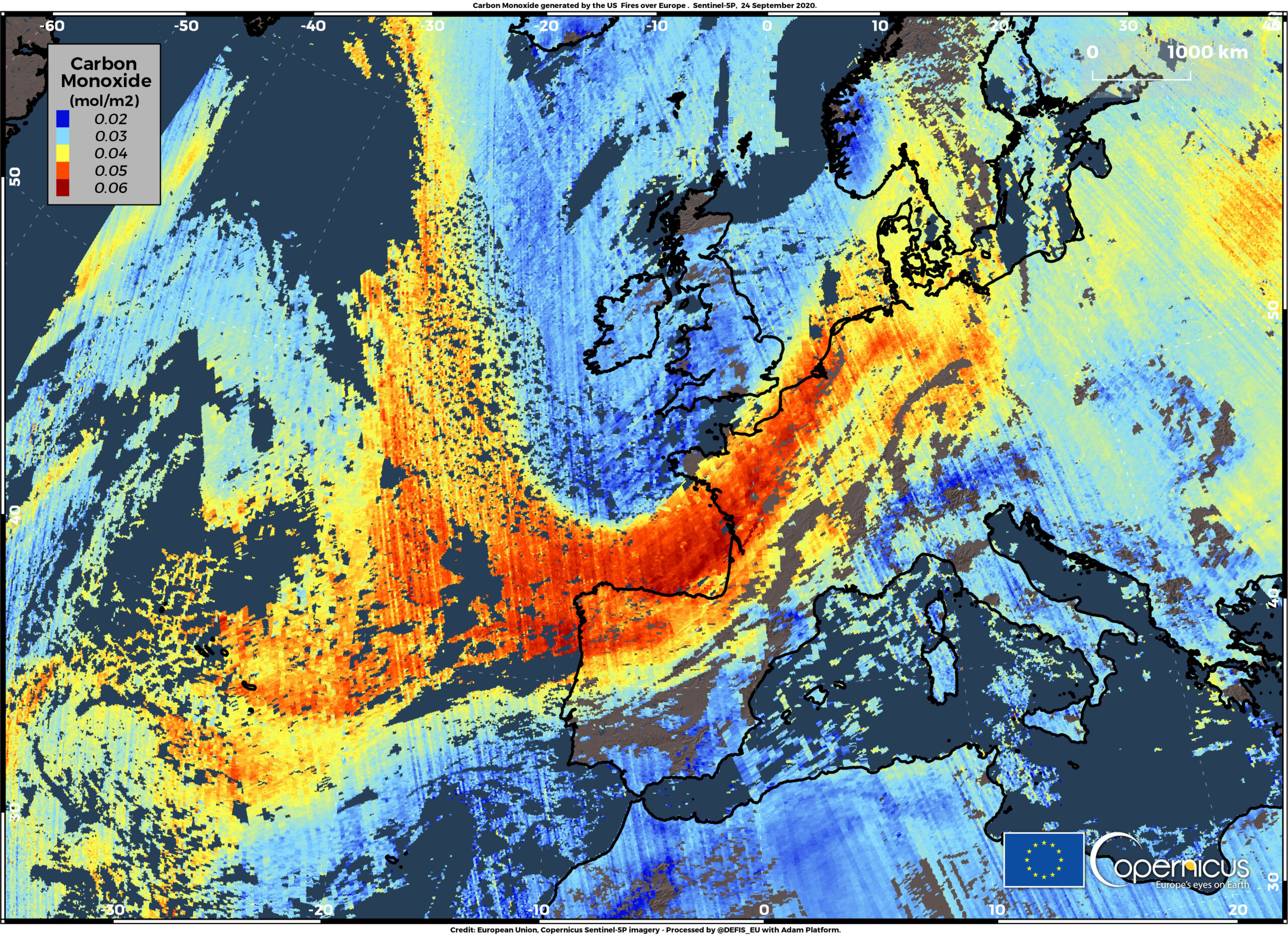
Carbon monoxide emission from forest fires on the West Coast of the USA reaching Europe – credit image credit European Union, Copernicus Sentinel-5P image
What is the relationship between carbon monoxide and wildfires?
Carbon monoxide (CO) forms as a by-product released during wildfires, alongside fine particulate matter (PM2.5). It also contributes to intensifying fires indirectly by accumulating in the air. This leads to a deterioration in air quality in surrounding areas and affects the regenerative capacity of forests by weakening surviving trees. Additionally, biodiversity is lost as species are forced to migrate or face local extinction.
What regulations exist to control carbon monoxide in the atmosphere?
Many governments and international organisations have implemented strict regulations to control CO emissions and exposure. These primarily focus on vehicle emissions and industrial facilities.
Moreover, air quality standards ensure monitoring to maintain safe exposure levels for human health. Various agencies set exposure limits:
World Health Organisation (WHO):
- 90 parts per million (ppm) over 15 minutes.
- 25 ppm over 1 hour.
- 10 ppm over 8 hours.
Occupational Safety and Health Administration (OSHA), USA:
- Workplace exposure limit: 50 ppm average over an 8-hour shift.
- Any reading above 10 ppm indicates an unusual CO source that should be investigated.
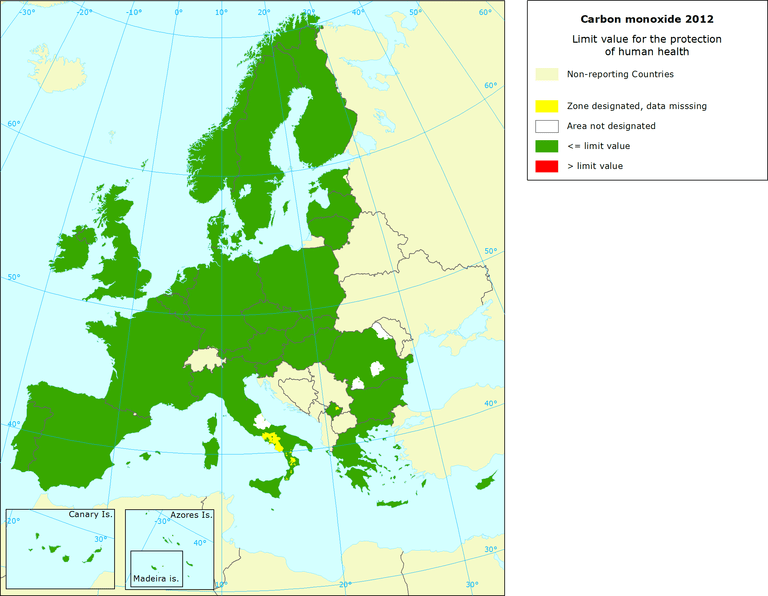
Carbon monoxide (CO) limit value for human health protection – image credit EEA
European Environment Agency (EEA) and European Commission (EU):
- Daily limit value: 10 mg/m³ (8.6 ppm) as the maximum daily 8-hour average.
In conclusion, it is essential that authorities and citizens are informed and committed to ensuring a healthy and safe environment through environmental monitoring and the enforcement of these regulations.
What technologies exist to reduce carbon monoxide emissions?
To reduce CO emissions into the atmosphere, vehicles using fossil fuels are equipped with three-way catalytic converters that convert CO into carbon dioxide before it is released. Engines, in general, benefit from more efficient combustion systems that promote complete fuel burning.
Switching to renewable energy sources (wind, solar, and hydroelectric) reduces dependency on fossil fuels, thereby lowering emissions of CO and other air pollutants.
In industries, waste gas incinerators use a thermal oxidation process to convert CO into carbon dioxide before it is released into the atmosphere.
Why is carbon monoxide monitoring important?
Air quality monitoringControlling air quality is an essential task in order to enjoy optimal environmental conditions for healthy human development and to keep the environment i...
Read more is essential to ensure public safety. It also helps prevent health issues and protect the environment. Since carbon monoxide is an invisible gas and a silent killer, the continuous operation of air quality monitoring systems is crucial for the early detection of CO.
Early detection allows for quick and effective control of dangerous CO levels before they cause harm to human health and ecosystems. In high-traffic areas, it helps prevent mass poisoning incidents by enabling informed decision-making based on real-time CO sensor data.
These data also help to identify CO exposure patterns in urban and industrial areas, supporting preventative mitigation actions. Cities can use these patterns to comply with regulations, plan public mobility policies, and promote cleaner energy sources. Furthermore, CO monitoring data enhance transparency in public information, boosting citizen confidence in environmental policies.
CO monitoring also enables preventative action in environmental emergencies such as wildfires and helps protect both firefighters and nearby residents from CO exposure.
Similarly, in industrial facilities, early CO detection through continuous monitoring ensures rapid identification of potential leaks.
What do carbon monoxide monitoring systems provide?
Advanced CO sensors, strategically installed in monitoring areas, such as those developed by Kunak, detect this dangerous gas in real time.
Kunak sensors, equipped with a proprietary algorithm, calculate CO concentration without requiring external reference data, ensuring measurement accuracy. Additionally, an interconnected perimeter network allows data to be analysed via digital platforms like Kunak AIR Cloud. This enables quick, informed decision-making by detecting CO presence and triggering alerts when thresholds are exceeded.
These sensors help urban planners design preventative models and implement action plans, such as improving tunnel ventilation or increasing green spaces, to reduce CO presence.
Kunak’s smart cartridges integrate high-precision electrochemical sensors to detect and measure CO and other gases, meeting the monitoring and control needs of industrial facilities, natural environments, and cities. These devices also ensure long-term measurement stability, minimising the need for frequent adjustments and recalibrations.
Kunak has implemented solutions in various settings to monitor and control carbon monoxide. For example, the Valparaíso metro in Chile continuously monitors multiple pollutants, including CO, to improve air quality in the underground system.
In Libya, LIFECO has deployed Kunak’s air quality sensorMeasuring air quality is essential for improving human and environmental health. Changes in the natural composition of the air we breathe are common in ind...
Read mores at its Marsa El Brega plant to protect workers and nearby communities, meeting stringent industrial requirements.
Another success case in air pollution control is the Low Emission Zone in Bilbao, Spain. Kunak’s strategic monitoring network uses 41 Kunak AIR Pro sensors to continuously and accurately assess air quality in the city.
In conclusion, Kunak employs advanced technologies and integrated solutions to measure and control carbon monoxide in the air, significantly contributing to air quality improvement and environmental protection.




