Hydrogen sulphide (H2S), also known as hydrosulphuric acid or sewer gas, is a gas unmistakable due to its characteristic rotten egg smell, noticeable even at very low concentrations. It is present in the atmosphere both due to natural sources (volcanic activity, hot springs and decomposition of organic matter) and industrial activities (wastewater treatment, pulp and paper production, fertilisers, and oil and natural gas refineries). It poses a significant health risk due to its high toxicity and its ability to disperse quickly through the air; a dangerous pollutant especially when exposure is prolonged. Once inhaled, H2S spreads through the bloodstream, systemically affecting various physiological functions.
What is hydrogen sulphide (H2S)?
Hydrogen sulphide, or H2S, is a simple molecule made up of two hydrogen atoms and one sulphur atom, but its simplicity does not make it less significant. It is a colourless and extremely flammable gas with a very distinctive smell, similar to rotten eggs. It is also water-soluble and denser than air, meaning it can accumulate in low-lying or poorly ventilated areas.
One of its most notable properties is its high toxicity: even at low concentrations it can cause adverse effects on human health. As its concentration in the air increases, its effects can become more severe, ranging from eye and respiratory irritation to loss of consciousness and, in extreme cases, death. Furthermore, the human sense of smell quickly becomes accustomed to H2S, making it even more dangerous as it becomes undetectable even if it remains in the environment.
It also exhibits interesting chemical behaviour industrially: it is a reducing agent, meaning it can easily react with other compounds, making it relevant in certain industrial processes, although handling it requires extreme caution.

Sewage channel
Where is H2S found?
H2S is a gas that can be naturally found in various environments: volcanic areas, sulphur-rich springs, emissions from the Earth’s crust through seabed cracks, swamps and stagnant waters, as well as in crude oil and natural gas.
But hydrogen sulphide is not only generated in nature: many human activities also produce it, such as sewer systems (hence the name “sewer gas”, as it forms in places where organic matter decomposes without oxygen), wastewater treatment plants, pig farms, fertiliser factories, and industries like paper and pulp. It is also emitted into the air from refineries, petrochemical plants, food processors, coke ovens and tanneries.
Air Quality Innovation in Just 1 Click
Stay informed about the air you breathe!
Subscribe to our newsletter to receive the latest updates on environmental monitoring technology, air quality studies, and more.
It is even produced inside the human body, specifically in the mouth and digestive system; certain bacteria generate H2S when breaking down proteins, both from plant and animal origin, contributing to bad breath.
Impact of hydrogen sulphide on health and the environment
Effects of H2S on human health
Hydrogen sulphide is a gas especially hazardous to public health for two main reasons: its high toxicity and its ability to become undetectable to the human nose after some time. While its rotten egg smell makes it easy to recognise at first, with prolonged exposure the sense of smell stops detecting it, which can create a false sense of security, particularly in industries like pulp and paper mills, wastewater treatment plants, landfills, oil and gas drilling and refining, rayon factories, animal farms, and cesspools.
Exposure to low concentrations of H2S may cause eye, nose, and throat irritation or worsen conditions such as asthma in those who suffer from it. As the level of the gas increases in the air, more serious symptoms can occur such as headaches, nausea, difficulty breathing and even loss of consciousness. In extreme cases and with high exposures, H2S can cause respiratory paralysis and lead to death within minutes. In some individuals, the health effects of H2S may become chronic, leading to memory and concentration problems, motor coordination issues, and persistent headaches.
This is why prevention is the best environmental defence. Risks must be addressed in advance in settings where H2S may be released, such as industrial facilities, wastewater and sludge treatment stations, or volcanic areas. It is advisable to monitor the air and use reliable and accurate H2S sensors, as well as provide appropriate protective equipment for workers. Moreover, staff training is essential: knowing how to act in the event of a leak of this toxic gas can be the difference between a scare and a tragedy.
To understand the health impact of H2S, it is useful to know its effects according to concentration (ppm or parts per million) in the air:
- 0.01–1.5 ppm: Noticeable smell (olfactory threshold).
- 2–5 ppm: Unpleasant odour; possible mild eye and throat irritation.
- 10–50 ppm: Eye and respiratory irritation; headache.
- 50–100 ppm: Loss of smell (anosmia); risk of pulmonary oedema.
- 100–200 ppm: Loss of consciousness within minutes; serious damage from prolonged exposure.
- >500 ppm: Death within minutes due to respiratory arrest (“sulphide shock”).
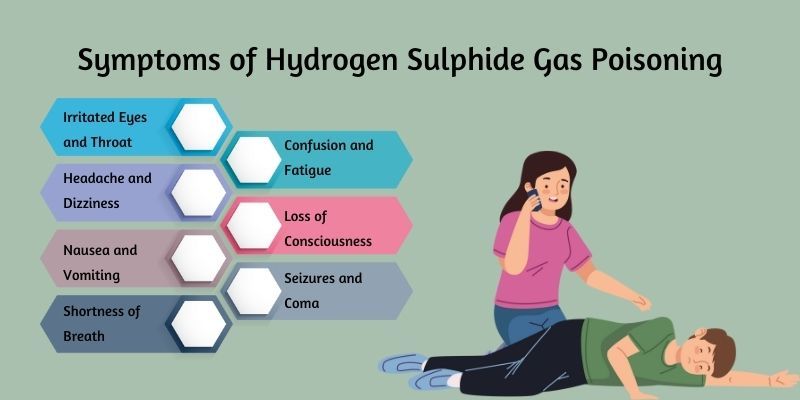
Symptoms of hydrogen sulphide poisoning by Canada Safety Training Centre
Environmental effects of H2S
Hydrogen sulphide is not only known for its unpleasant rotten egg smell, but also for its concerning environmental effects. When released into the air, particularly in industrial or geothermal areas, it contributes to air pollution. Although it disperses quickly in open spaces, in enclosed or poorly ventilated places it can accumulate and pose a risk by creating hazardous environments for both people and ecosystems.
When H2S builds up in stagnant water bodies, it can reduce oxygen levels, affecting aquatic life and accelerating the decomposition of organic matter. This disrupts ecosystem balance and endangers sensitive species.
Another worrying characteristic of hydrogen sulphide is its high corrosiveness. In humid environments, this gas reacts with metallic surfaces, speeding up their oxidation and deterioration. This presents a challenge for industries such as refineries, wastewater treatment plants, and underground tunnels, where maintenance becomes more complex and expensive due to the structural damage caused by its presence.
In summary, while often associated with public health, H2S also has a notable environmental impact. Proper management and control are crucial to protect biodiversity and are essential for preserving infrastructure from corrosion caused by its presence.

Workers carrying out operations in the sewerage area
Exposure limits and current regulations
Due to the serious effects that H2S has on human health, organisations such as the OSHA (Occupational Safety and Health Administration) and the NIOSH (National Institute for Occupational Safety and Health) in the United States, as well as the European Commission and agencies such as the ECHA (European Chemical Agency) and the EU-OSHA (European Agency for Safety and Health at Work), have established strict occupational and environmental exposure limits.
Below are the reference values and applicable regulations for occupational exposure:
| Region / Organisation | Limit type | Value | Duration / Note |
|---|---|---|---|
| United States – OSHA | PEL (TWA) | 10 ppm | 8-hour time-weighted average |
| PEL (STEL) | 15 ppm | Short-term exposure limit (15 minutes) | |
| PEL (Ceiling) | 20 ppm | Must not be exceeded at any time; escape mask required | |
| United States – NIOSH | REL (TWA) | 10 ppm | 10-hour time-weighted average |
| REL (STEL) | 15 ppm | Short-term exposure limit (15 minutes) | |
| IDHL | 100 ppm | Immediately dangerous to life or health; acute exposure may be fatal | |
| European Union – Directive 98/24/EC | VLA-ED (daily) | 5 ppm (7 mg/m³) | Daily average |
| VLA-EC (short term) | 10 ppm (14 mg/m³) | Short-term exposure (15 minutes) | |
| United States – ACGIH | TLV-TWA | 1 ppm (1.4 mg/m³) | 8-hour time-weighted average |
| TLV-STEL | 5 ppm (7 mg/m³) | Short-term exposure limit (15 minutes) |
*The American Conference of Governmental Industrial Hygienists (ACGIH), as an independent scientific organisation, conducts research on occupational risks and publishes updated recommendations which, although not legally binding, are highly regarded worldwide.
How hydrogen sulfide (H2S) is measured
The danger posed by the presence of H2S gas in the atmosphere makes its accurate measurement critical in industries such as oil, wastewater treatment, and mining. Below are described the most commonly used techniques for its determination:
Main measurement techniques
The measurement of hydrogen sulfide (H2S) can be approached through two broad categories of techniques that quantify based on:
Sensory techniques (subjective/human)
These are qualitative methods for H2S that rely on human olfactory perception as a tool. However, the applicability of these techniques based on olfactory receptors has significant limitations due to physiological and environmental factors such as olfactory system habituation, gas toxicity at high concentrations, and individual variability in perception. Together, they add uncertainty to the reproducibility of results, compromising their reliability as a standardized measurement method.
These sensory methods are based on:
▸ Dynamic olfactometry
- Method: Dilute a sample of contaminated air with a neutral gas so that the concentration of odorous compounds reaches the perception threshold. This dilution is exposed in a controlled manner to a panel of trained evaluators who identify the presence of the odor based on intensity and recognition.
- Application: Widely used in environmental impact studies and atmospheric emission control in industries such as wastewater treatment, organic waste management (composting), paper production, and refineries.
- Advantages: Reflects real human odor perception based on direct sensory responses. Useful for validating odor dispersion methods and estimating population annoyance thresholds.
- Limitations: Not a specific method for H2S detection (there may be a mixture of volatile compounds with similar odor properties). It is an imprecise technique for quantifying exact pollutant concentrations and does not provide continuous monitoring.
▸ Trained sensory panel
- Method: Conducts a direct air qualityAir quality refers to the state of the air we breathe and its composition in terms of pollutants present in the atmosphere. It is considered good when poll...
Read more assessment through a group of people trained to identify and characterize specific odors. These evaluators follow standardized protocols to record intensity, frequency, and persistence of odors in specific environments. They work in the field where odorous compounds are emitted, without requiring sample extraction or prior processing. - Advantages: It is a fast, low-cost technique useful for spot evaluations. Requires no instrumentation or laboratory conditions, facilitating implementation in diverse environments.
- Limitations: Highly subjective, not precisely quantifiable, and not applicable in high-toxicity environments with dangerous pollutant concentrations.
Instrumental techniques (objective/technological)
These are the most suitable for measuring actual H2S concentrations, both spot and continuous. They are based on high-sensitivity measurement systems:
▸ Electrochemical methods
- Operation: Based on the measurement principle of the chemical reaction between H2S and specific electrodes. This process generates an electric current proportional to its ambient concentration. Data from these electrochemical sensors, like those integrated in Kunak AIR stations, provide quantitative measurement in concentration units.
- Application: Widely used in environmental monitoring of urban and industrial environments such as atmospheric surveillance infrastructure control networks, in industrial sectors like wastewater treatment plants, refineries, and chemical plants. Also integrated as portable devices in personal protective equipment (PPE) for occupational safety in risk areas.
- Advantages: Low cost as an analytical technique and compact size (integratable into portable devices) combined with good sensitivity at ultra-low levels and operational capability for continuous measurement, ideal for numerous environmental applications.
- Limitations: Sensors may degrade over time, limiting their lifespan, and environmental variations (humidity and temperature) may affect their stability and reliability, requiring frequent calibrations.
▸ Absorption spectrometry (UV, FTIR, DOAS)
- Operation: Based on gases’ ability to absorb electromagnetic radiation at specific wavelengths. The method uses a light beam directed through a gaseous sample and, by measuring the amount of radiation absorbed at certain spectral bands, identifies and quantifies H2S presence. Depending on the electromagnetic spectrum wavelength used, it can be ultraviolet (UV), Fourier transform infrared spectroscopy (FTIR) if using mid-infrared, or differential optical absorption spectroscopy (DOAS) which enables remote detection by absorbing light in open atmospheres.
- Application: In industrial emission control for specific production processes and chimneys for real-time emission supervision, in laboratory analysis for high-precision gas mixture determination, and during remote monitoring over large distances.
- Advantages: High precision allows H2S quantification with great accuracy, even at very low concentrations, avoiding interference. Highly suitable for validating pollutant dispersion models and environmental impact assessments.
- Limitations: Both instrumentation and operation are very costly, complex to operate as they require specialized personnel and rigorous periodic calibrations to ensure optimal performance.
▸ Gas chromatography with specific detection (GC-FPD or GC-SCD)
- Operation: Advanced analytical technique where specific gaseous compounds like H2S are separated and identified by detecting their physicochemical properties through a mobile phase (carrier gas) and stationary phase (chromatographic column). Then, using a selective detector like flame photometric (GC-FPD) or sulfur chemiluminescence (GC-SCD), H2S is precisely identified and quantified.
- Application: Suitable for environmental laboratory analyses in industrial facilities requiring precise gas characterization. Also useful for determining composition and proportion of volatile compounds in complex gas samples.
- Advantages: High precision and specificity (without interference from other gaseous compounds) allows exact H2S quantification.
- Limitations: Not suitable for continuous field measurement. Its laboratory-restricted application makes it an economically costly method in use and maintenance. Also complex to use as operation requires specialized personnel for data interpretation and analytical system maintenance.
▸ Colorimetric techniques (Dräger tubes, detection kits)
- Operation: Method based on the chemical reaction of the gas with a specific reagent causing a gradual color change depending on its concentration. Dräger detector tubes and other colorimetric kits contain a medium impregnated with H2S-sensitive reagents that activate upon gas exposure.
- Application: Used for quick spot measurements in locations with potential H2S presence like industrial plants, sewer systems, and wastewater treatment plants. Suitable for occupational safety when immediately verifying H2S presence in confined spaces before personnel entry.
- Advantages: Simplicity requires no calibration or advanced knowledge for handling. Compact, easily transportable devices providing immediate measurements facilitating operational decision-making.
- Limitations: Doesn’t perform continuous real-time measurement and visual estimation of color change adds quantification variability, making it an inaccurate measurement method. Also has restricted shelf life as reagents degrade over time, altering measurement reliability.
▸ Metal oxide sensors (MOX)
- Operation: Use the variation in electrical conductivity of a semiconductor material when exposed to H2S. The metal oxide surface changes upon gas interaction, and this is proportional to the hydrogen sulfide concentration present.
- Advantages: Low-cost technique with high durability and operation capability in different environmental situations. Can be integrated into low-power monitoring systems.
- Limitations: Low selectivity as the metal sensor may be altered by other ambient gases. Also sensitive to environmental factors like temperature and humidity, as well as other chemical compounds, requiring frequent calibration to improve data reliability.
Comparative table of key measurement techniques
| Technique | Type | Continuous | Accuracy | Specific to H2S | Cost |
|---|---|---|---|---|---|
| Dynamic olfactometry | Sensorial | ✘ | ✘ | ✘ | Medium |
| Sensory panel | Sensorial | ✘ | ✘ | ✘ | Low |
| Electrochemical sensor | Instrumental | ✔ | ✔ | ✔ | Low |
| Spectroscopy (FTIR, UV) | Instrumental | ✔ | ✔✔ | ✔ | High |
| Gas chromatography GC-FPD | Instrumental | ✘ | ✔✔✔ | ✔✔ | Very High |
| Colorimetric tubes | Instrumental | ✘ | ✔ | ✔ | Low |
| MOX sensor | Instrumental | ✔ | ✘ | ✘ | Very Low |
Advantages of connected sensors and continuous monitoring
Monitoring hydrogen sulphide using connected sensors enables real-time data collection even in remote locations and transmits this data for integration and analysis on web platforms. These platforms include tools that support decision-making and help mitigate the risks associated with the presence of H2S.
They can be used in a wide variety of industrial and professional settings as they do not require a mains power supply, being powered by solar panels. They are also resistant to ambient humidity thanks to their IP68 certification, indicating a high level of protection against dust and water.
Thanks to their use of wireless communication technology, sensor networks are considered the most suitable measurement tools for remote areas that need low power consumption and long-range coverage, for urban environments with high-speed connectivity, or for local industrial plant networks.
Connected sensor networks for measuring H2S represent the future of industrial safety. By combining technology, data, and automation, they not only detect the danger of hydrogen sulphide but also prevent disasters through the use of collective intelligence.
Monitoring H2S with advanced solutions such as Kunak AIR
Monitoring hydrogen sulphide is essential to ensure air quality and prevent health and environmental risks. Kunak’s advanced solutions stand out in this field thanks to their high-precision electrochemical sensor technology, capable of detecting even minimal variations in ambient H2S concentration.
The competitive advantage of Kunak’s H2S monitoring system is its ability to operate in real time, allowing for the early identification of pollution events and supporting timely decision-making to mitigate their impact. Additionally, by integrating the data into analysis platforms, it enables the optimisation of environmental management, making Kunak an ideal option for industrial emissions control and protecting nearby communities.
Detection of mercaptans
Furthermore, Kunak’s technology for continuous real-time monitoring of odorous substances such as H2S in the air can even detect methyl mercaptans, a very common substance in industrial environments. These are strong-smelling, unpleasant organic compounds, similar to alcohols but with sulphur instead of oxygen. Thanks to the H2S electrochemical sensors integrated into Kunak devices, mercaptans can be detected — which, however, often go unnoticed by other measurement methods such as traditional H2S analysers.
Sensor-based gas monitoring systems are effective tools for the monitoring and management of odorous compounds, providing critical information for implementing corrective measures and improving air quality.
Use cases and sectors that measure H2S
Cerro Patacón landfill (Panama)
The Cerro Patacón landfill, located near Panama City, is one of the most relevant use cases for H2S measurement developed by Kunak in the specific field of pollutant gas measurement. This landfill manages over 40% of the country’s generated waste, so practices like intentional waste burning have caused serious environmental pollution problems, affecting air quality and consequently the health of nearby communities.
To mitigate this severe environmental impact, a network of Kunak AIR Lite stations was deployed, capable of measuring in real-time suspended particles (PM) and pollutant gases such as methane (CH4) and hydrogen sulfide (H2S). Additionally, these stations record wind speed and direction, facilitating the identification of pollution sources and prediction of emission dispersion.
The benefits have been immediate and notable by providing continuous, autonomous monitoring of odor pollution and particles (thanks to solar power) that enables developing an early warning system to notify authorities when critical levels are exceeded. This way, public health protection is permanently guaranteed for nearby communities against toxic gases and harmful particles in the air.
Meat industry in Des Moines (Iowa, United States)
In Des Moines, capital of Iowa state, the persistent problem of odor pollution causing over 200 annual citizen complaints about unpleasant odors from three industrial meat plants led local authorities to install 10 Kunak AIR Pro odor monitors at strategic points around the identified emitters, along with a weather station at the Municipal Services Center.
These devices measure in real-time the concentration of hydrogen sulfide (H2S), ammonia (NH3) and volatile organic compounds (VOCs), providing accurate air quality data. The result has been control of odor-causing pollutants through continuous monitoring, a data-driven quantitative approach to establish odor thresholds and require corrective measures from emitting companies, and a significant improvement in environmental management by reducing and mitigating the impact of odor pollution on the local community.
Wastewater treatment in Rishon LeZion (Israel)
In Israel, the Shafdan wastewater treatment plant (WWTP), located in Rishon LeZion, due to hydrogen sulfide (H2S) emissions, was causing odor nuisance in nearby residential areas.
This environmental issue was addressed by deploying a network of 7 Kunak AIR stations around the plant perimeter. This network has enabled continuous monitoring of pollutant gases, wind speed and direction, temperature, relative humidity and atmospheric pressure. Additionally, an H2S analyzer was installed in a chimney to calculate emission plume dispersion and determine which areas exceed the odor threshold, verifying or dismissing neighborhood complaints.
Continuous and validated measurement of H2S levels around the treatment plant perimeter has been achieved, establishing an early warning system and defining greater precision in identifying odor sources, differentiating whether H2S comes from the WWTP or other nearby industrial emission sources. This is how local authorities respond more efficiently to odor emissions, reducing environmental impact and improving quality of life for residents near the facility.
Frequently asked questions (FAQs) about hydrogen sulphide (H2S)
What does hydrogen sulphide smell like?
If you ever smell rotten eggs, you’re probably in an environment where hydrogen sulphide (H2S) is present in the air. It’s such a distinctive scent that it can be easily detected even at low concentrations. Naturally, it originates from the decomposition of sulphur-containing organic matter, such as in wastewater or swamps.
However, be cautious — it can also be emitted during industrial processes and, at higher concentrations, it has a harmful effect on health. Worst of all, when inhaled over prolonged periods at high concentrations, it causes olfactory fatigue in our nasal receptors, which increases the risk of poisoning as the distinctive smell stops being perceived.
Is hydrogen sulphide dangerous?
Indeed, hydrogen sulphide is a very dangerous gas because at high concentrations it can be lethal. Even at low levels, it can cause irritation, dizziness, and respiratory problems. While its rotten egg smell makes it easily recognisable, with prolonged exposure our noses stop detecting it, as it acts like an olfactory anaesthetic, making it impossible to notice even when it’s present in high amounts.
Where can H2S be found in industry?
Hydrogen sulphide (H2S) is a colourless gas that, even though we can’t see it, is more common than we might think. It is found in nature — in places like volcanoes, sulphurous springs, and stagnant waters — but we also generate it through our activities: sewer systems, pig farms, chemical industries, oil and gas extraction and refining, tanneries, paper and pulp mills, rayon factories, fertiliser plants, and wastewater treatment facilities.
Even our own bodies produce it, particularly in the mouth and digestive tract, contributing to unpleasant bad breath. Although its recognisable smell might seem harmless, its build-up can be dangerous, which is why H2S detection and control are essential in industrial and urban settings.
How can the presence of H2S be detected?
The presence of hydrogen sulphide (H2S) can be detected in several ways, depending on the setting and the level of precision needed. While its intense and characteristic rotten egg smell is easy to recognise, at high concentrations it becomes undetectable even though it’s still present. That’s why it’s important to have sensors for this dangerous pollutant gas. To measure it, electrochemical sensors are the most widely used, as they offer accurate real-time readings.
In situations where rapid detection is required, reactive strips that rely on a chemical reaction can be a temporary solution. However, continuous monitoring with advanced, specialised sensor technology is the most reliable solution, as it provides real-time detection, enables alert generation when thresholds are exceeded, and helps prevent exposure risks.
What regulations apply to H2S in workplace or urban environments?
Due to the severe health risks posed by the presence of H2S, authorities such as the OSHA (Occupational Safety and Health Administration) and the NIOSH (National Institute for Occupational Safety and Health) in the United States, and the European Commission with agencies like ECHA (European Chemical Agency) and EU-OSHA (European Agency for Safety and Health at Work), have set strict exposure limits for both workplace and environmental settings.
Additionally, the American Conference of Governmental Industrial Hygienists (ACGIH), as an independent scientific organisation, investigates occupational hazards and publishes updated recommendations that, while not legally binding, are highly regarded worldwide.
Conclusion
Mitigating the risks of exposure to H2S gas requires the use of advanced technology to ensure proactive prevention against one of the most dangerous gases in industrial and urban settings. Invisible and deadly at high concentrations, it can also cause adverse effects even at low levels. Its presence in refineries, wastewater treatment plants, or sewers demands not only strict protocols but also technological solutions capable of anticipating risks.
Continuous monitoring is no longer optional — it is essential.
Devices such as Kunak sensors, precise, reliable, and adaptable, enable real-time H2S detection, even in extreme conditions. They support an immediate response to protect both workers and nearby communities. And thanks to their integration with data analysis platforms, they become a critical tool capable of predicting risk scenarios and optimising preventive measures. Because when it comes to H2S, the best strategy is not to underestimate it.




