Invisible yet powerful: ammonia (NH3) is a colourless gas which, although naturally present in the atmosphere in small amounts, can become an unwelcome enemy when released into the air, causing intense irritation to the eyes, throat and skin. However, far from being only a threat, this compound plays a key role in multiple industrial processes, where its versatility makes it an indispensable ally. It is associated with numerous processes in the chemical industry and, above all, is linked to agriculture, as NH3 is an essential component in the manufacture of nitrogen fertilisers.
It is also a gas released in livestock production through ammonification or the microbiological decomposition of nitrogen present in excreta—a process responsible for most ammonia emissions to air.
Additionally, in recent decades, ammonia has emerged as an atmospheric pollutant of growing concern due to the critical role it plays in forming secondary fine particles (PM2.5). Because its gas density is about 0.59 times that of air, it is lighter and tends to disperse rapidly in the atmosphere. It therefore reacts with other air pollutantsAir pollution caused by atmospheric contaminants is one of the most critical and complex environmental problems we face today, both because of its global r...
Read more such as sulphur dioxide (SO2)Sulphur dioxide (SO2) is a colourless gas with a pungent odour that causes an irritating sensation similar to shortness of breath. Its origin is anthropoge...
Read more and nitrogen oxides (NOₓ), generating hazardous ammonium sulphate and ammonium nitrate aerosols.
For all these reasons, in this article we explain how ammonia has become one of the most relevant emerging air pollutants, impacting both human health and the environment. We examine how its importance has led to close inclusion and oversight in occupational regulations and its incorporation into pollution-control strategies. We highlight the importance of environmental monitoring and how proper detection directly improves air qualityAir quality refers to the state of the air we breathe and its composition in terms of pollutants present in the atmosphere. It is considered good when poll...
Read more and environmental protection.
Ammonia (NH3) is one of the principal forms of reactive nitrogen in Europe, with significant impacts on air quality, ecosystem eutrophication and biodiversity. Its emissions are largely linked to agriculture, and efficient management is essential to meet environmental objectives and protect public health. Sutton, M. A., et al. (2013). The European Nitrogen Assessment: Sources, Effects and Policy Perspectives. Cambridge University Press.
What is ammonia (NH3) and where does it come from?
Ammonia is a gas with a penetrating, unpleasant odour and a strong irritant capacity, causing burning sensations, itching and watering of the eyes, nose, throat and skin. It is a nitrogen chemical compound consisting of a molecule with one nitrogen atom covalently bonded to three hydrogen atoms. Understanding the chemical and physical properties of this molecule is key to grasping its impact on air quality and to designing effective environmental mitigation policies.
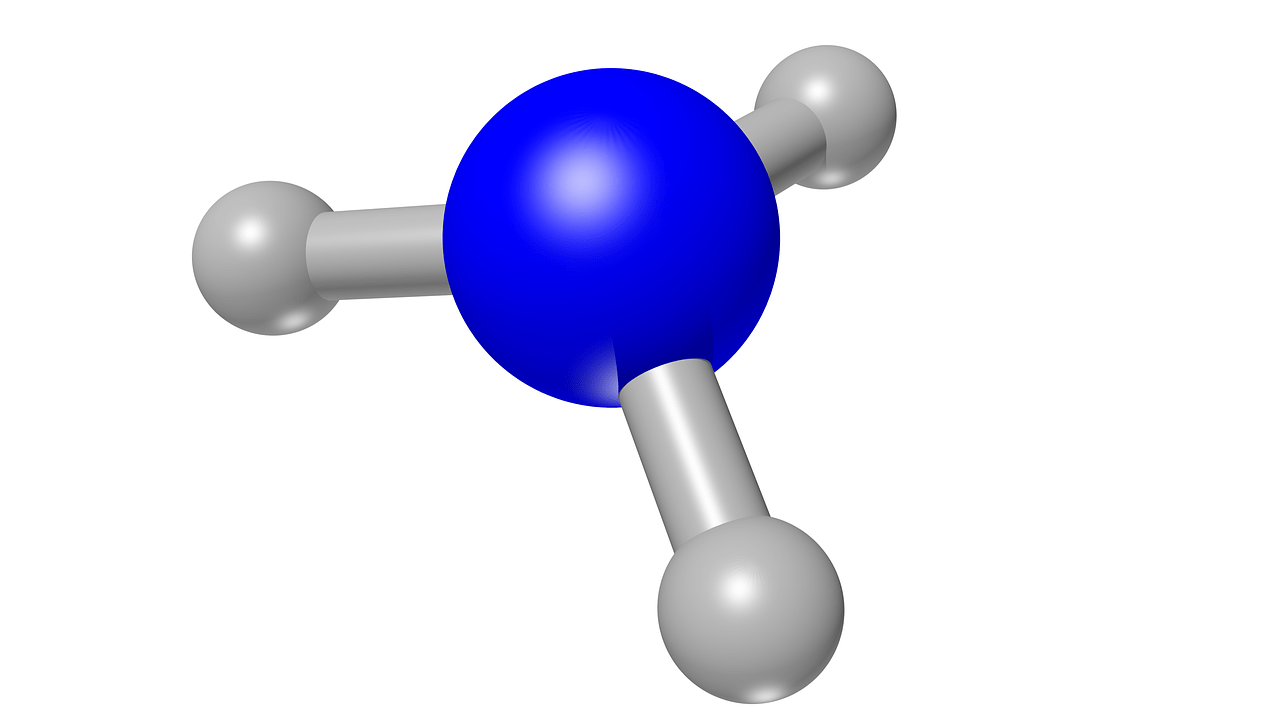
The ammonia molecule is composed of one nitrogen (N) atom bonded to three hydrogen (H) atoms.
Air Quality Innovation in Just 1 Click
Stay informed about the air you breathe!
Subscribe to our newsletter to receive the latest updates on environmental monitoring technology, air quality studies, and more.
Chemical and physical properties of NH3
At ambient temperature NH3 remains a colourless gas, though it has a very pungent, unpleasant odour that can be detected even at very low ambient concentrations (5 ppm).
Its high polarity underpins its main chemical property: it is highly soluble in water, which particularly favours hydrogen-bond formation. It thus forms ammonium hydroxide (NH4OH), a weak base that maintains an alkaline pH in solution, and readily combines with acids to form salts. This is how it reacts with hydrochloric acid to form ammonium chloride and with nitric acid to form ammonium nitrate—salts that make up an important fraction of fine particulate matter (PM2.5). In addition, ammonia can evaporate easily; being lighter than air, it disperses rapidly through the atmosphere.
From an atmospheric perspective, ammonia is a fundamental precursor gas in chemical reactions that lead to the formation of secondary particles, in particular ammonium sulphate and ammonium nitrate aerosols, principal components of fine particulate matter (PM2.5). These reactions occur when NH3, as a weak base, neutralises acidic compounds such as sulphur dioxide (SO2) and nitrogen oxides (NOₓ), facilitating the formation of solid particles that contribute to air pollution and affect health and the environment.
Main sources of ammonia emissions
Although ammonia is naturally present in the atmosphere at low concentrations, its primary origin lies in the microbiological degradation of organic nitrogen compounds derived from plant and animal waste. As organic matter decomposes naturally, the gas is gradually released into the atmosphere.
However, anthropogenic activities—such as the use of nitrogen fertilisers in agriculture; increased manure loads; industrial processes requiring the synthesis of chemical products (refineries, plastics, pharmaceuticals and explosives); municipal waste in landfills; and leaks in sewer networks and wastewater treatment—cause its atmospheric share to multiply, placing NH3 among today’s principal emerging pollutants.
From a quantitative perspective, and according to recent European Union data, around 94% of ammonia emissions come from the agriculture and livestock sector. The most relevant sources within this sector are the use of nitrogen fertilisers (especially urea and anhydrous ammonia) and the management of manure and slurry, whose degradation releases large volumes of NH3 into the atmosphere. Guidance documents for the measurement and modelling of new air-quality pollutants. Ammonia (NH3). Ministry for the Ecological Transition and the Demographic Challenge. Government of Spain.
Effects of ammonia on human health
The corrosive and irritant properties, together with the penetrating odour of NH3, mean that its inhalation or direct exposure in the environment can cause discomfort and harm, with the severity of effects (irritation of the eyes, nose and respiratory tract) depending on the concentration and duration of exposure.
Acute and chronic exposure
Chronic exposure to atmospheric ammonia poses a serious risk to respiratory health, especially in occupational or environmental settings where gas concentrations exceed levels considered safe. Unlike acute exposure, which typically causes immediate symptoms such as eye or nasal irritation, prolonged and repeated inhalation of this compound can trigger irreversible physical and pathological changes.
Continued contact of NH3 with the bronchial epithelium induces a persistent inflammatory response, promoting thickening of the bronchial walls, airway hyperreactivity and progressive obstruction of airflow.
One of the most concerning clinical outcomes associated with such exposure is chronic obstructive pulmonary disease (COPD), a degenerative condition that includes chronic bronchitis and pulmonary emphysema. Affected patients often present symptoms such as persistent cough, shortness of breath and a feeling of chest tightness. In addition, structural damage to the pulmonary alveoli compromises gas exchange, which can lead to chronically low blood oxygen and a significant reduction in lung capacity.
Vulnerability of sensitive populations
Furthermore, the constant irritation of the upper and lower airways caused by NH3 weakens local immune barriers, increasing vulnerability to recurrent respiratory infections such as acute bronchitis or pneumonia; a phenomenon that is especially relevant among sensitive populations such as children, older people and those with pre-existing respiratory diseases.
Consequently, chronic exposure to ammonia not only impairs lung function but also increases the risk of serious respiratory diseases, with clinical implications that may require prolonged pharmacological treatment, pulmonary rehabilitation and even ventilatory support in advanced stages.

Extensive livestock farming is one of the sources of ammonia emissions into the air.
Environmental impact of ammonia
The major environmental significance of ammonia lies in its widespread emission into the atmosphere as a result of intensive agricultural and livestock practices (especially the use of nitrogen fertilisers and the management of livestock waste) as well as industrial processes.
Its presence in air not only affects atmospheric quality but also plays a fundamental role in the formation of another major air pollutant: fine particles (PM2.5), whose suspension in air has direct implications for human health and climate balance.
Therefore, understanding the behaviour of ammonia in the atmosphere is essential to design effective mitigation and adaptation policies to address environmental challenges and planetary sustainability.
Contribution to air pollution
In the atmosphere, ammonia readily reacts with nitrogen oxides (NOₓ) and sulphur oxides (SOₓ) to form particulate matter (PM2.5). These fine particles, with a diameter below 2.5 micrometres, can remain suspended for days and travel long distances.
The presence of PM2.5 in air not only degrades air quality but is directly associated with public health problems: when inhaled, particles penetrate deep into the respiratory system, increasing the risk of conditions such as chronic bronchitis, asthma and allergies, and are even linked to cases of premature mortality.
Ammonia is an important air pollutant due to its contribution to the formation of fine particles (PM2.5), which pose a risk to public health. Ammonia exposure should be controlled to protect human health, as elevated levels can cause respiratory irritation and long-term damage. World Health Organization (2000). Air Quality Guidelines for Europe.

Frog in eutrophic waters.
Effects on ecosystems and biodiversity
Environmentally, the long-range transport and deposition of PM2.5 contribute to the acidification and eutrophication of soils and water bodies, altering their chemical and biological balance.
This reduces the natural buffering capacity of ecosystems and significantly affects nutrient availability. The process limits the growth of certain plant species while favouring others, altering vegetation composition and ultimately reducing and reshaping existing biodiversity.
In aquatic ecosystems, excess nitrogen derived from ammonia can trigger eutrophication, characterised by excessive growth of phytoplankton and algae. This leads to a depletion of dissolved oxygen, resulting in the loss of aquatic fauna and a general deterioration in the ecological quality of natural aquatic environments.
Regulations and concentration limits for ammonia
Having a regulatory framework for the presence of ammonia in ambient air and in workplaces is key to protecting public health and sustainability. However, despite the environmental relevance of NH3 and its potential health impact, regulation shows significant differences between ambient and occupational exposures.
International regulations (WHO, EU, EPA)
The World Health Organization (WHO), the European Union (EU) and the United States Environmental Protection Agency (EPA) address the control of ammonia mainly because of its role as a precursor of fine particles (PM2.5) and the indirect effects it has on air quality. Traditionally, there have been no specific limit values for ammonia in ambient air; however, its monitoring and measurement are included in recent regulations due to its impact on secondary pollutants.
In the EU, with the implementation of Directive 2024/2881 (in force since 11 December 2024), ambient air quality control and assessment are strengthened, bringing legal values closer to WHO guidance; it also consolidates the periodic review and adjustment of these values in line with the latest scientific evidence.
Although the Directive sets stricter limits for other pollutants and promotes improvements to environmental monitoring systems, there is still no specific ambient threshold for ammonia in air, other than its indirect consideration as a PM2.5 precursor.
Ammonia (NH3) emissions in the European Union fell by only 16% between 2005 and 2022. The main source is agriculture, responsible for 93% of emissions, primarily through manure management, livestock housing and fertiliser application. Despite current policies, several Member States will not meet their 2020–2029 emission-reduction commitments, and additional measures are required to reach the 2030 targets. EEA (2022). European Environment Agency Report on Air Pollution and NH3 emissions.
In the United States, the EPA regulates ammonia mainly for its effects as a hazardous substance in accidental releases, and there is ongoing debate over whether to consider it an indicator to assess ambient air conditions.
Inhalation exposure to ammonia can cause respiratory irritation and long-term non-carcinogenic effects. The toxicological review suggests that although ammonia is produced naturally in humans and animals, prolonged inhalation of elevated concentrations can trigger inflammatory responses in the respiratory system, affecting lung function and overall health. US EPA (2016). Integrated Science Assessment for Oxides of Nitrogen, Oxides of Sulfur, and Ammonia.
Occupational exposure limit values (OELs)
In the workplace, regulation is much more precise. In Spain and across the European Union, the occupational exposure limit (OEL) for ammonia commonly lies around 20–25 ppm as an 8-hour time-weighted average, following European and national recommendations.
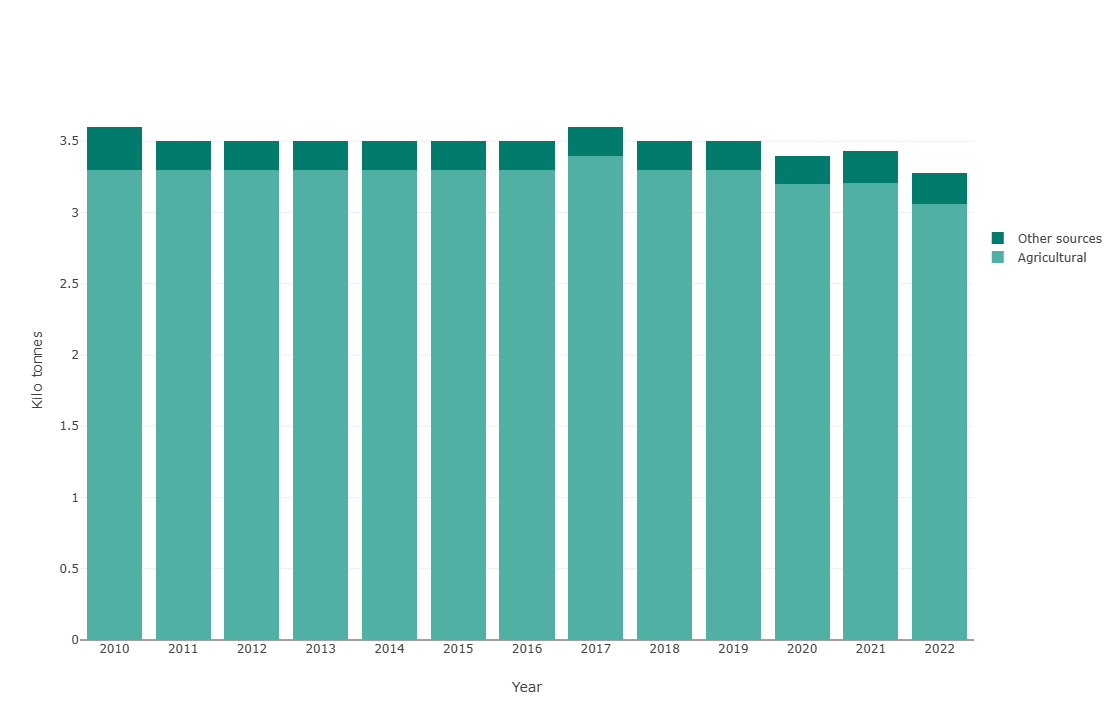
Comparison of ammonia emissions from the agricultural sector versus the share from other sources.
In the United States, regulation sets a permissible exposure limit according to OSHA of 50 ppm averaged over an 8-hour working day, while bodies such as NIOSH recommend 25 ppm and the ACGIH also proposes similar time-weighted values (TWA) over an 8-hour shift.
Taken together, these values protect workers against the acute and chronic effects of ammonia in industrial environments, and stand in marked contrast to the absence of limits for the general population in ambient air.
Measuring and monitoring ammonia in air
Ammonia plays a fundamental role in many industrial, agricultural and livestock processes, but it is also a pollutant that affects air quality and human health. Detecting and quantifying it accurately with a technical solution that is practical and easy to deploy—such as environmental monitoring—is essential. Monitoring a wide range of environments with optimised NH3 detection helps to comply with environmental regulations, minimise emissions and protect exposed people.
Traditional measurement methods
For many years, environmental measurement of atmospheric ammonia has relied mainly on passive and analytical techniques:
- Passive tubes and chemical samplers: absorb the gas over a set period and are then analysed in a laboratory to estimate accumulated concentrations.
- Chromatography: the reference method, capable of separating and quantifying NH3 with high accuracy; usually carried out in specialised laboratories.
While reliable for spot sampling or baseline studies, these methods have important limitations:
- High costs for analysis and logistics.
- Low temporal resolution, as they do not provide real-time variation.
- The need to transport samples and wait for results.
Therefore, in many situations they are not suitable for agile emissions management or for delivering the continuous monitoring required by current regulations.
Advanced NH3 sensors and continuous monitoring
The most up-to-date solutions for detecting and controlling ammonia are based on direct, real-time measurement technologies, such as calibrated electrochemical sensors integrated into environmental monitoring stations.
One example is the Kunak’s smart cartridge for NH3, designed to:
- Detect low concentrations with high selectivity.
- Maintain stable calibration to ensure data quality.
- Enable full traceability of every measurement thanks to automatic logs.
- Operate with remote control, allowing adjustments and diagnostics without travelling to the installation point.
Its technology offers unique advantages over traditional methods:
- Accurate, continuous measurements with minute-level temporal resolution.
- Real-time alerts for rapid response to concentration peaks.
- Integration with data-management systems, enabling advanced analysis and correlation with other environmental variables.
This approach positions Kunak solutions not merely as control tools but as strategic allies for industry, public authorities and research projects seeking to measure and manage ammonia efficiently, precisely and in a connected way.

Occupational safety sign warning of possible ammonia presence in the air.
Frequently asked questions about ammonia (NH3)
What is ammonia and why is it considered an air pollutant?
Ammonia (NH3) is a colourless gas composed of nitrogen and hydrogen that occurs naturally in the atmosphere at very low concentrations. However, when released in large quantities by human activities (use of nitrogen fertilisers in intensive agriculture, intensive livestock farming and numerous industrial processes), it becomes a significant air pollutant.
Although not as toxic as some other industrial gases, ammonia indirectly contributes to the formation of fine particles (PM2.5) by reacting with other compounds in the air, such as nitrogen oxides and sulphur dioxide. These particles can penetrate deep into the lungs, affecting the respiratory and cardiovascular health of exposed populations. In addition, its accumulation in sensitive ecosystems can disrupt soil and water balance, promoting phenomena such as eutrophication.
For these reasons, ammonia is regarded as an emerging air pollutant in air-quality and environmental-sustainability policies.
What are the main sources of ammonia emissions?
Ammonia (NH3) is naturally present in the atmosphere through the microbiological decomposition of plant and animal organic matter. However, the most significant emissions come from human activity, especially the intensive use of nitrogen fertilisers, manure management, and various industrial processes such as the production of plastics, pharmaceuticals or explosives. Landfills and wastewater treatment plants also contribute, making NH3 one of today’s most notable emerging air pollutants.
How does ammonia affect human health?
Due to its corrosive and irritant properties, ammonia (NH3) can cause discomfort and harm when inhaled, from eye and nasal irritation to more severe respiratory injuries, depending on concentration and exposure time.
Acute exposure to NH3 causes immediate irritation of the eyes, nose and throat, while prolonged or chronic exposure can lead to persistent bronchial inflammation and progressive impairment of lung function. This situation favours the development of chronic obstructive pulmonary disease (COPD), characterised by persistent cough, breathlessness and reduced oxygen exchange in the lungs.
Children, older adults and people with respiratory diseases are particularly vulnerable, as constant irritation weakens their defences and promotes recurrent infections. Long-term exposure to ammonia may require prolonged medical treatment and can significantly affect quality of life.
What is the relationship between ammonia and PM2.5 particles?
Ammonia plays an essential role in the secondary formation of fine particles (PM2.5). When released into the atmosphere, it reacts with nitrogen oxides and sulphur dioxide to generate ammonium salts—ammonium nitrate and ammonium sulphate—which constitute a significant fraction of fine particulate matter.
Because of their small size, these particles can penetrate deep into the lungs and reach the bloodstream, increasing the risk of respiratory and cardiovascular diseases. Controlling NH3 emissions is therefore crucial not only to reduce gaseous pollution but also to mitigate PM2.5 formation and protect public health.
How is ammonia measured in air-quality monitoring?
Ammonia in air can be measured using passive and active methods, which differ in precision and temporal resolution. Passive tubes and chemical samplers provide average concentrations over long periods and are useful for background studies or specific campaigns, although they do not offer real-time data.
By contrast, continuous monitoring with calibrated electrochemical sensors, such as those integrated into Kunak AIR Pro systems, provides direct, traceable measurements with high temporal resolution. This technology enables constant tracking of NH3 concentrations, early detection of peaks and the efficient management of emissions in urban, agricultural and industrial environments.
Conclusion
Ammonia is one of today’s most relevant air pollutants, particularly because of its role in the formation of fine particles (PM2.5) and their impact on human health and ecosystems. Ammonia is closely linked to agricultural, livestock and industrial activities—settings where emissions control is key to reducing pollution and complying with European environmental regulations.
In this context, continuous, accurate monitoring of NH3 has become a strategic necessity. Advanced technologies, such as those integrated into Kunak AIR Pro, allow ammonia and other gases to be measured with high temporal resolution, full traceability and complete connectivity, providing environmental managers with reliable data for decision-making and impact prevention.
Today, thanks to smart solutions that are easy to integrate in any environment, it is possible to move from spot sampling to comprehensive air-quality control, optimising emissions management through continuous monitoring.




